Follicular dendritic cell sarcoma of the liver with metachronous small bowel and splenic metastases: a case report and literature review
Introduction
Follicular dendritic cells (FDCs) are immune cells found in germinal centres of lymphoid follicles in secondary and tertiary lymphoid organs. They originate from mesenchymal cell lines instead of hematopoietic ones, despite appearing similar to true dendritic cells due to their filiform dendritic processes. They play an integral immunological role in the presentation of antigens to B cells and assistance in their maturation.
Primary sarcomas of the liver are extremely uncommon, representing less than 0.1% of all primary hepatic tumors (1). Follicular dendritic cell sarcoma (FDCS) of the liver is especially rare, given that only 30% of all FDCS tumors occur in extranodal sites (2). This report highlights an unusual metachronous presentation of a primary liver FDCS with its imaging findings and management.
Case presentation
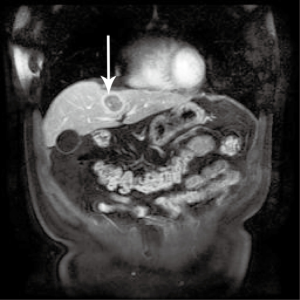
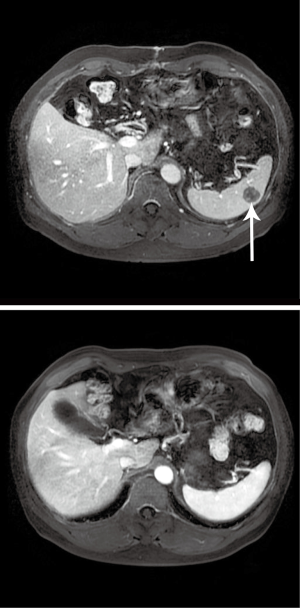
A 50-year-old gentleman with a history of stage III nasopharyngeal carcinoma (NPC) status post chemoradiotherapy. On follow-up surveillance 18 months later, magnetic resonance imaging (MRI) scan revealed a 3.1 cm × 3.1 cm rim-enhancing lesion with central necrosis in segment 4a of the liver (Figure 1). A positron emission tomography (PET) scan was subsequently performed to exclude other possible sites of metastasis. It revealed the same liver lesion with avid fluorodeoxyglucose (FDG) uptake and no other lesions detected. The maximum standard uptake value (SUVmax) of this lesion was 12.2. The impression was of a single NPC metastatic lesion in the liver. After a multidisciplinary tumor board discussion, given the patient’s fitness for surgery, long disease free interval and it was a solitary liver lesion, resection would be appropriate. The patient subsequently underwent left hemi-hepatectomy and cholecystectomy due to its proximity to the left portal vein. Recovery was smooth and uneventful. The patient was discharged on post-operative day (POD) 5.
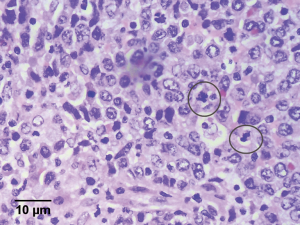
Histological examination was performed and reviewed by a gastrointestinal histopathologist (KHT Lim). It revealed a 3 cm × 2 cm × 2 cm soft, circumscribed tumor in segment 4 of a mildly steatotic liver, 1.7 cm from the parenchymal resection margin and 0.5 cm from the liver capsule. It exhibited pathological features characteristic of FDCS. The tumor composed of irregular nests of large, epithelioid cells with indistinct cytoplasmic borders, vesicular, bilobed and multiple nuclei with prominent nucleoli (Figure 2). Immunohistochemical (IHC) staining was strongly positive for FDC markers such as CD21, fascin (strongly), CD35 and clusterin (weakly) (3,4). They were also positive for Epstein-Barr virus (EBV) by in situ hybridization. They were, however, negative for S-100 and CD30, making melanoma, epithelioid peripheral nerve sheath tumors, interdigitating dendritic cell tumors and anaplastic large cell lymphoma (monomorphic variety) less likely. Numerous cytokeratins (AE1AE3, MNF-166, CAM 5.2, OSCAR, 34BE12) and epithelial membrane antigen were also negative, making carcinoma less likely. Infiltrating reactive lymphocytes were positive for CD3 and CD20. After a multi-disciplinary tumor board discussion, given that there is limited evidence in the literature regarding the efficacy of adjuvant chemotherapy post-curative resection for hepatic FDCSs, the consensus was there was little role for further adjuvant therapy and the patient should be closely followed up with 6 monthly surveillance imaging.
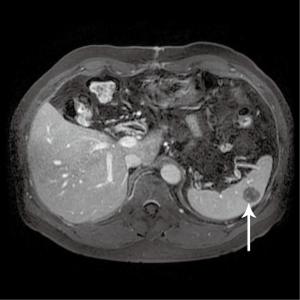
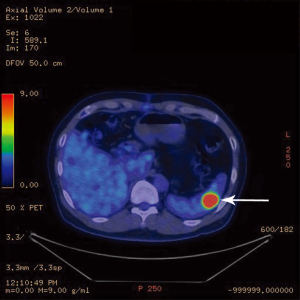
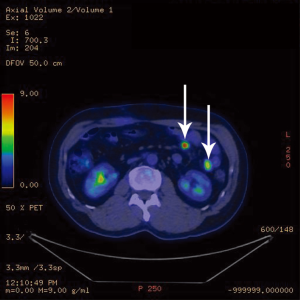
A follow-up computer tomographic (CT) scan 6 months after the initial hepatectomy revealed a new 1.4 cm lesion in the spleen (Figure 3) and two soft tissue bowel masses in the jejunum (4.1 cm) and distal ileum (7.1 cm), associated with enlarged mesenteric nodes (0.7 and 2.2 cm). These lesions were FDG avid as well on a PET scan. The SUVmax in the spleen was 14.2 (Figure 4), mesenteric nodes 12.1 and small bowel 6.3 (Figure 5). These lesions were not detected during staging scans prior to his hepatic resection (Figure 6).
The patient subsequently presented with melena and hemoglobin level of 5.8. He underwent Tc-99m labelled red blood cell scintigraphy, which revealed intermittent gastrointestinal tract bleeding from the distal ileum. He received 3 pints of packed cell transfusion (PCT) and subsequently underwent an emergency laparotomy to stop the gastrointestinal bleeding. Concomitant resection of small bowel revealed three discrete lesions located in the proximal jejunum (1 cm × 1 cm), distal jejunum (5 cm × 5 cm) and distal ileum (6 cm × 6 cm). Lesions showed blood clots, confirming the source of recent bleeding.
Histological examination revealed plaque-like, ulcerative and infiltrative lesions measuring 6.0 cm × 10.5 cm × 2 cm (distal ileum), 1.0 cm × 2.2 cm (proximal jejunum) and 4.3 cm × 4.8 cm × 4.9 cm (distal jejunum). The tumors were well circumscribed with heaped up brown borders, a central area of necrosis and multiple regions of hemorrhage. Adjacent bowel mesentery contained multiple matted hard white nodules suspicious for satellite tumor deposits. Pathologic findings for all 3 tumors suggested FDCS with a similar IHC profile as the previous liver lesion. Impression was that of primary FDCS of the liver with small bowel and splenic metastases.
The patient tolerated the operation well. The post-operative course was unremarkable and he was discharged on POD 6. Capecitabine was started as adjuvant chemotherapy after his second surgery. He has been on 6-monthly follow-up since. Yearly CT scans and 6-monthly blood test results confirmed that the splenic lesion has been stable, with no new focus of metastasis identified since.
Discussion
FDCS of the liver is a very rare entity, first reported in 1996 by Shek et al. (4) and with only 17 reported cases in the English literature to date (1,4,5-12). Clinical, pathological and surgical features of these cases with complete histological and IHC details were reviewed and summarized (Table 1).
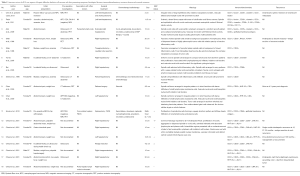
Full table
Primary malignant neoplasm of lymph nodes showing features of FDCS were first recognized as a distinct entity in itself after being reported by Monda et al. in 1986 (13). It only accounts for 0.4% of all soft tissue sarcomas, but has been reported to exhibit significant recurrent and metastatic properties (3). FDCSs commonly occur in lymph nodes (cervical and axillary), with only 30% of all recorded cases occurring in extra-nodal regions (2,14). Common extra-nodal sites involved include the oral cavity, spleen, liver, small bowel, pancreas and peritoneum (2,3). The disease commonly presents as large mass with mean sizes of 7–10 cm and appears to affect a relatively young population, with a median age at presentation of 41 years (2,5). Most of the intra-abdominal tumors reported are significantly larger and more aggressive than their extra-abdominal counterparts with the most frequent sites of metastasis being the lung, liver and lymph nodes (5,6).
Approximately 20% of cases appear linked to hyaline vascular type Castleman disease, which has been widely regarded as a precursor of FDCS. About 12% of all cases of FDCS tumors are known to be associated with EBV infection. The role of EBV, however, remains only postulative (3,10,15,16).
Preoperative diagnosis of FDCS is based on clinical examination, imaging and pathologic assessment. Imaging is mainly used to identify the extent of tumor invasion as well as for staging purposes. Ultrasound, CT and MRI are the usual modalities used, though PET is used occasionally to detect occult lesions. A well-defined mass with regional lymphadenopathy, homogenous enhancement and internal necrosis are CT findings characteristic of intra-abdominal FDCS (6). Histologically, FDCs are large, binucleate, and form clusters with lymphocytes. They are best visualized with immunostaining using the FDC markers CD21, CD35, R4/23, clusterin, and KiM4p (3). Pathognomonic evidence of FDCS includes microtubuloreticular structures (MTRS) and markedly increased levels of intracellular clusterin (17). Clusterin is the marker most commonly stained to provide strong grounds for suspicion of FDCS (4,9).
In this patient, the hepatic lesion was detected in his 6-monthly surveillance imaging due to his history of NPC, while the splenic and small bowel lesions were absent, even on retrospective review. Only on follow-up imaging with CT and PET scans, after his liver resection, were the new lesions in the small bowel and spleen discovered. Retrospectively, chronologically and as supported by literature thus far, it is consistent that the segment 4a liver lesion is a primary liver FDCS with subsequent metastases to the small bowel and spleen. Nonetheless, given that small bowel mesenteric primary FDCS is more common than primary liver FDCS (2), the possibility that the primary lesions originated from the small bowel or its mesenteric lymph nodes, but was too small to be detected by pre-operative imaging and missed during the exploratory laparotomy at the first surgery should be noted. We think this less likely as the initial PET scan clearly did not detect any other FDG avid regions besides the solitary liver lesion.
Due to the rarity of primary hepatic sarcomas, the prognostic factors, natural history and optimal treatment strategy is poorly characterized. The current gold standard for treatment of FDCS of the liver is complete surgical resection. This is, however, associated with a local recurrence rate of 23% and subsequent metastatic spread in 21% of cases (5). Previous studies of hepatic FDCS have yet to report on any distant metastasis to date. Post-operatively, the ‘CHOP’ regime has been recognized as a modality of adjuvant therapy for prevention of recurrence, if any at all. It consists of cyclophosphamide, doxorubicin, oncovin/vincristine and prednisolone (1,5,18). Still currently on clinical trials, data regarding efficacy and validity of the ‘CHOP’ regime is extremely limited. Compilation of case reports of all FDCSs regardless of location reveal a 5-year post-operative recurrence-free survival rate of 27–32% and a 5-year overall survival rate of 79% (5,14).
It has been suggested that hepatic FDCS might not be as aggressive as its other intra-abdominal counterparts, behaving more like a low-grade soft tissue sarcoma (5). Fonseca et al. reported that local recurrence in hepatic FDCSs occurred in 3 out of 15 cases over a variable period of follow-up (6 months to 5 years). This is in contrast to a recurrence rate of 12 of 31 patients with FDCS regardless of the intra-abdominal organ involved (2). Among the above-mentioned 15 cases of hepatic FDCS, there is predilection for females and almost all are EBV-positive (including our patient). All except 1 patient underwent surgical resection and had a mean survival of more than 2 years. Recently, Li et al. reviewed all 106 reported cases of extranodal FDCS. It was reported that surgery is the most common treatment (94.3%) and a third of the patients (35 of 106) underwent for post-operative adjuvant therapy (chemotherapy or both radiation and chemotherapy). Primary chemotherapy was used in only 2.8% of the patients and none of patients treated with primary chemotherapy showed a response (14). A larger size of tumor and high mitotic counts (more than 5 per 10 high power fields) were found to be strongly associated with recurrence. The role and efficacy of adjuvant treatment is poorly documented and unclear, but is generally reserved for tumors recalcitrant to surgical resection, as neo-adjuvant therapy in preparation of surgical resection, recurrence or incompletely resected lesions.
Conclusions
This is the first report of a case of a primary liver FDCS with metachronous spleen and small bowel metastases that presented with gastrointestinal bleeding. We found that in this case, PET/CT is a good modality to detect and survey for recurrent FDCS at close intervals (3–6 months) post-treatment. A multi-disciplinary tumor board should be the basis for management and decision-making especially in such rare tumors. This disease remains a rare but important differential diagnosis of liver tumors, both for its potential of complete curative surgical resection as well as the known significant risk of recurrence. While other therapeutic modalities are explored with the advancement of chemotherapy and radiotherapy, surgical resection remains the mainstay of therapy. Further studies are required to understand the natural history and the metastatic potential of primary liver FDCS as well as explore novel effective adjuvant therapy to improve the outcome of these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Martins PN, Reddy S, Martins AB, et al. Follicular dendritic cell sarcoma of the liver: unusual presentation of a rare tumor and literature review. Hepatobiliary Pancreat Dis Int 2011;10:443-5. [Crossref] [PubMed]
- Fonseca R, Yamakawa M, Nakamura S, et al. Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma: a review. Am J Hematol 1998;59:161-7. [Crossref] [PubMed]
- Chan JK, Fletcher CD, Nayler SJ, et al. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer 1997;79:294-313. [Crossref] [PubMed]
- Shek TW, Ho FC, Ng IO, et al. Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells. Am J Surg Pathol 1996;20:313-24. [Crossref] [PubMed]
- Shinagare AB, Ramaiya NH, Jagannathan JP, et al. Primary Follicular Dendritic Cell Sarcoma of Liver Treated With Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone Regimen and Surgery. J Clin Oncol 2011;29:e849-51. [Crossref] [PubMed]
- Shek TW, Liu CL, Peh WC, et al. Intra-abdominal follicular dendritic cell tumour: a rare tumour in need of recognition. Histopathology 1998;33:465-70. [Crossref] [PubMed]
- Torres U, Hawkins WG, Antonescu CR, et al. Hepatic follicular dendritic cell sarcoma without Epstein-Barr virus expression. Arch Pathol Lab Med 2005;129:1480-3. [PubMed]
- Bai LY, Kwang WK, Chiang IP, et al. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol 2006;36:249-53. [Crossref] [PubMed]
- Selves J, Meggetto F, Brousset P, et al. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am J Surg Pathol 1996;20:747-53. [Crossref] [PubMed]
- Chen TC, Kuo TT, Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol 2001;14:354-60. [Crossref] [PubMed]
- Tsunemine H, Akasaka H, Kusama T, et al. Hepatic follicular dendritic cell sarcoma favorably controlled by transcatheter arterial chemoembolization. Intern Med 2010;49:2703-7. [Crossref] [PubMed]
- Cheuk W, Chan JK, Shek TW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 2001;25:721-31. [Crossref] [PubMed]
- Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 1986;122:562-72. [PubMed]
- Li L, Shi YH, Guo ZJ, et al. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol 2010;16:2504-19. [Crossref] [PubMed]
- Biddle DA, Ro JY, Yoon GS, et al. Extranodal follicular dendritic cell sarcoma of the head and neck region: three new cases, with a review of the literature. Mod Pathol 2002;15:50-8. [Crossref] [PubMed]
- Horiguchi H, Matsui-Horiguchi M, Sakata H, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Pathol Int 2004;54:124-31. [Crossref] [PubMed]
- Ono Y, Terashima K, Liu A, et al. Follicular dendritic cell sarcoma with microtubuloreticular structure and virus-like particle production in vitro. Pathol Int 2009;59:332-44. [Crossref] [PubMed]
- Nicolini A, Mancini P, Ferrari P, et al. Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC). Biomed Pharmacother 2004;58:447-50. [Crossref] [PubMed]


