Updates in Mirizzi syndrome
Introduction
In 1948, Pablo Luis Mirizzi, an Argentine surgeon, described “the hepatic duct syndrome” in the context of cholecystitis and cholelithiasis. Mirizzi postulated that the common hepatic duct obstruction was quite functional, and the mechanical obstruction of the gallbladder and the consequent inflammatory process of the infundibulum predisposed to the contraction of a “muscular sphincter” located in the common hepatic duct. Nowadays, it is well known that there is no sphincter in the hepatic duct. Jaundice, the main clinical manifestation, is the result of external compression caused by the impacted calculus (1-15).
However, the partial duct obstruction secondary to an impacted calculation and the associated inflammatory process, was first described in 1905 by Kehr and 1908 by Ruge (1,2,7,10,16).
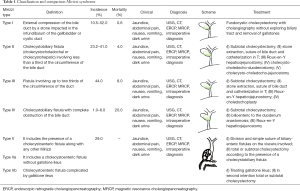
In 1982, McSherry et al. classified the Mirizzi syndrome in two types, based on the findings of the endoscopic retrograde cholangiography: type I involved a partial or complete external obstruction of the common hepatic duct by a stone impacted in the cystic duct or the Hartmann pouch, resulting in inflammation of the Calot triangle. Type II referrers to the formation of a communication between the cystic duct and the common hepatic duct, it is accompanied by a cholecystocholedochal fistula caused by an eroded stone in the common duct (6,9,13,15,16). Later in 1989, Csendes et al. classified the Mirizzi syndrome in four types which categorized cholecystocholedochal fistulas based on the degree of destruction. In 2007, Csendes adds a further type to the classification which was validated by Beltran, this classification is summarized in Table 1 (1,2,4-6,8,9,12,14-21).
Full table
Mirizzi syndrome, known as extrinsic bile compression syndrome, is a rare complication of cholecystitis and chronic cholelithiasis secondary to obliteration of the infundibulum of the gallbladder (Hartmann’s pouch) or cystic duct caused by the impact of one or more calculations in these anatomical structures. It causes compression of the adjacent bile duct, resulting in partial or complete obstruction of the common hepatic duct, and finally triggering liver dysfunction. It is accompanied by inflammation of the gallbladder, and in some cases cholecystocholedochal fistula may be present (Mirizzi syndrome type II, III, IV) or not (Mirizzi syndrome type I) (1,3,5-7,9,11-14,16,18,20,22-26).
Mirizzi syndrome is uncommon, showing an incidence of less than 1% per year in developed western countries and from 4.7% to 5.7% in developing countries. The incidence reported in Mexico is 4.7%. There is not an established difference between males and females. It prevails between the fourth and seventh decade of life (2,7,8,12-14,21,24,25).
The incidence increases in patients undergoing cholecystectomy to 0.3–5% and 0.1–2.7% among patients with cholelithiasis; meanwhile, patients with gallbladder cancer have the highest risk (>25%) (1,4-15,17-20,23,26).
Objectives
Define the actualities concerning the Mirizzi syndrome from the definition, classification, and diagnostic approach because it is currently an untouched topic due to its low incidence and prevalence.
Emphasize the importance and various methods to diagnose Mirizzi syndrome. Despite its low frequency, it is very important an early diagnosis in the patient’s prognosis, significantly reducing the risks, and complications.
Describe the actualities in different ways to approach Mirizzi syndrome and their advantages and disadvantages.
Methods
We made a research in various databases; Medline, Cochrane, Embase, Medscape, PubMed, in order to gather information and clinical evidence, based on different articles published over a period of 18 years [1997–2015], using keywords in Spanish and English: background, incidence, pathophysiology, diagnosis and treatment of Mirizzi syndrome, surgical approach, laparoscopic approach, and using filters Spanish and English, the year 1997–2015.
Results
Mirizzi syndrome can be originated by an inflammatory process secondary to erosion caused by an impacted gallstone in the gallbladder infundibulum or Hartmann’s pouch and cystic duct (1,10,21,25,26).
The inflammatory process favors the formation of adhesions, by merging their walls by inflammatory edematous tissue that eventually will become fibrotic to the neighboring structures; most frequently the common bile duct, duodenum and colon (1,2,6,19,21,26).
Adherence to the bile duct contributes to external compression of the bile duct, leading to obstructive jaundice (2,26).
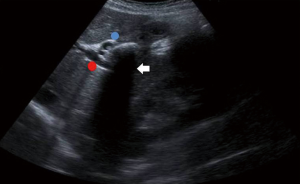
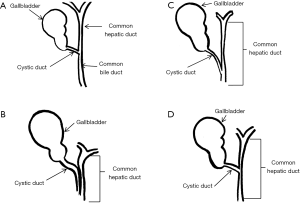
The impacted calculus along with the inflammatory response causes the external bile duct obstruction, and eventually the mucosa will erode progressing into a cholecystocholedochal or cholecystohepatic fistula that presents different degrees of communication between the bile duct and gallbladder (1,2,6,7,10,15,18-20,26). Ultrasound of Mirizzi syndrome in Figure 1. Anatomic variations of the cystic duct, represented in Figure 2, are common and predispose to the development of this syndrome, it occurs in 18–23% of cases (1,3,11,16). The distortion of the anatomy and the presence of a cholecystocholedochal fistula, increases the risk of bile duct injury during cholecystectomy (10), in Table 2 the variety of anatomical features found in Mirizzi syndrome is summarized (2,16).
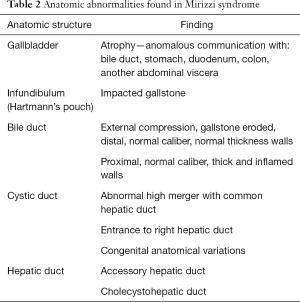
Full table
Unfortunately there are no pathognomonic signs, but it has been found that obstructive jaundice is the most common presentation (50–100%). It is often accompanied by pain in the right upper quadrant (50–100%) or epigastric pain, fever, nausea, vomiting, dark urine (62.5%), chills, tachycardia and anorexia (1-5,7,10-14,19,20,24-26). They can also occur in the context of acute cholecystitis, pancreatitis, and choledocholithiasis (1,4).
An early and accurate diagnosis has a major impact on management, morbidity, mortality, and preventing future complications by reducing them to 54% (6,8,10,18,25). However, in most patients, making a preoperative diagnosis with certainty can be difficult, achieving only 8% to 63.4% of cases. This condition can be confused with choledocholithiasis, and cholangitis due to the inflammation and adhesions in the Calot’s triangle. Also, an aberrant anatomy can mimic this condition making difficult to diagnose to the surgeon (2,9-11,14,23,25). The diagnosis of Mirizzi syndrome is based on clinical features, a high index of suspicion, or surgical intuition, which can be supplemented with radiologic imaging and endoscopic procedures, where ultrasound and endoscopic retrograde cholangiopancreatography (ERCP) stand as auxiliary diagnostic (1,2,16,26).
The laboratory results most often reports a high levels of bilirubin, alkaline phosphatase (ALP) and transaminase (1,4). However, if the preoperative diagnosis is not performed, intraoperative recognition and proper management is essential (10,16,26). CA 19-9 is a glycoprotein expressed by several cancers, including the biliary tree (95%), stomach (5%), colon (15%), liver (hepatocellular carcinoma 7%) and lung (13%); as well as normal pancreas, bile ductal epithelial cells, salivary mucosa, and meconium (17,20). However, levels can be elevated in many benign conditions such as patients with benign biliary tract disease usually have less than 1,000 U/mL levels (17,20,27).
Recently, high levels of the tumor marker CA 19-9 (>20,000 U/mL) were consistently found in patients with type II Mirizzi or higher grades of the syndrome (2,17). Nevertheless, there are few reported cases and it is considered that CA 19-9 is not useful as a screening test because of its low sensitivity in early stage disease, and hyperbilirubinemia also seems to be a confounder factor due it is associated with higher levels of CA 19-9 (2,17,20,27). Early diagnosis of Mirizzi syndrome is crucial for the patient’s prognosis and this can be achieved through ultrasound, computed tomography, ERCP, and magnetic resonance cholangiopancreatography (MRCP). Through these studies we can find the main features of Mirizzi syndrome: adhesions between the gallbladder and common bile duct, in the Calot’s triangle, extrinsic narrowing of the common hepatic duct, cholecystocholedochal fistula, a gallstone in the cystic duct and dilatation of the intrahepatic and common hepatic ducts (1-3,6-15,18,25,26,28).
Table 3 describes in detail the findings, advantages, disadvantages, sensitivity and specificity of each study available for Mirizzi syndrome diagnosis, including ultrasound (1-3,5,7,8,19,20,25,26) computed tomography (1-3,6,7,9,10,14,25,26), ERCP (1,2,6,8,10,12-15,18,19,26,28) and MRCP (6,8,10,11,14); it is noteworthy that ERCP and MRCP are considered the gold standard for diagnosing Mirizzi syndrome.
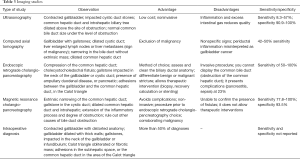
Full table
Nevertheless, more than 50% of patients with Mirizzi syndrome are diagnosed during surgery (4,7,10). Intraoperative ultrasound has proven to be a useful diagnostic tool to identify the anatomy of the biliary tree, and helps to perform a precise dissection of bile duct in an inflamed area. Also, it is recommended to be use at any moment, especially when there is no preoperative diagnostic confirmation, or even when Mirizzi syndrome is suspected during operation (2,4,10).
The differential diagnosis should include other causes of obstructive jaundice such as: gallbladder carcinoma (GBCa), cholangiocarcinoma, pancreatic cancer, metastatic disease, and sclerosing cholangitis (1).
The Csendes classification categorizes the cholecystobiliary fistula according to the degree of destruction of bile ducts. It is important to recognize and know this new subdivision since the extension of the cholecystobiliary fistula can have big implications in the way it is surgically manage (1,2,4,6,10,19).
The traditional treatment for Mirizzi syndrome has been surgery, resolving most cases. However, 8–25% of patients may require repair biliary tract by fistulization of the main bile duct (1,4,8,9,18).
The most common surgical approach is an incision in the fundus of the gallbladder and removal of impacted calculus (through a different incision on common bile duct or common hepatic duct). It is recommended a careful dissection of biliary structures, visualize the common bile duct (during surgery, if possible, or by postoperative ERCP), define the type and location of the fistula, relieve the obstruction, repair the defect, and ensure adequate drainage of bile (1,4,5,14,18,19,28).
When intraoperative diagnosis is made, cholangiography must be through a Kehr tube after removing any gallstone of decompressed gallbladder (2,6).
In all cases, it should be a frozen section of the gallbladder wall to rule out coexistent cancer (4). During surgery, the dissection of the Calot’s triangle can lead to injury of the bile duct, excessive bleeding, and other morbidities, such as sepsis, delayed biliary stricture, and secondary biliary cirrhosis (2). For complicated cases in which a fistula is present, it is suggested to perform a cholecysto-choledocus-duodenostomy, also it can be attempt the direct repair of the fistula by placing a T-tube or by choledochoplasty, overlapping the remaining gallbladder to close a cholecystocholedochal fistula, or Roux-en-Y hepaticojejunostomy to the inflammatory destruction of the common hepatic duct to prevent complications such as the formation of leaks, cholangitis, and biliary stricture. Extensive erosions of the bile duct are better managed with a biliary-enteric anastomosis (1,4,6,8-12,14,16,18,28,29).
Baer et al. have advocated for an anastomosis between the partially resected gallbladder (5 mm) and duodenum, which they called “cholecysto-choledocho-duodenostomy”, claiming satisfactory results and reduced risk of bile duct injury (1,2,23). A variation of the Baer anastomosis is the “cholecysto-choledocho-jejunostomy” proposed by Safioleas et al. (2).
It has been reported that laparoscopic subtotal cholecystectomy and laparoscopic cholecystectomy “fundus first” reduces the risk of injury to the bile duct and conversion rate to open surgery (10). Open surgery allows the use of proprioception or touch of the surgeon’s hand, and is generally accepted as a way to improve the safety in cases of severe inflammation. However, it is associated with long-term morbidity, and a difficult surgery is not necessarily easier or safer when performed open (5). In addition to this Csendes et al., reported a morbidity rate of 13% for patients with Mirizzi syndrome type I and II treated with open surgery, and it is significantly higher for patients with type III and IV of Mirizzi syndrome (18).
The surgical treatment of Mirizzi syndrome varies according to its type, in Table 1 are summarized. In general, endoscopic management includes biliary drainage of the common duct with or without drainage of the gallbladder by inserting the stent and stone removal by using a basket, balloon, mechanical, and electro-hydraulic lithotripsy or sphincterotomy (1,5,6,10,15,18,28).
In 1992, Paul et al. reported the first successful laparoscopy surgery as a treatment for Mirizzi syndrome type I, which were confirmed by other authors in different years, including Karademir et al. (1-3,5,6,8,9,11,17-19,23).
Analysis performed by Antoniou et al. showed that the procedure is associated with an overall complication rate of 16% and an overall mortality rate of 0.8%. The most common complications were residual stones and bile duct injury, but is noteworthy that these rates refer to Mirizzi syndrome type I and II (18,26).
Despite the successes reported, several authors mention that laparoscopic cholecystectomy can be technically difficult and dangerous for the bile duct because adhesions and severe inflammation in the Calot’s triangle can become dangerous if dissection is attempted. Therefore, laparoscopic cholecystectomy for Mirizzi syndrome currently cannot be recommended as a standard procedure due to the lack of studies and the controversial results of different reports (2,4,5,8-13,16,18,19,23).
Although the laparoscopic treatment is still not considered as standard treatment, with the right skills and equipment, it could be for some cases of type I and II of Mirizzi syndrome (6,11,12,21,28).
Proposed interventions to improve laparoscopic success includes: intraoperative cholangiography, and intraoperative ultrasound to delineate the biliary anatomy before dissection, it can also replicate the approach “fundic traction” which is used in open surgery, by using a liver so the Calot’s triangle can be more easily evaluated (5,8,21).
Therapeutic intervention is possible during ERCP, whether it can used as a primary treatment or as an adjunct to surgical treatment, since it has been reported better results after preoperative biliary drainage for cholangitis or jaundice, and when combined with duodenoscopy, laparoscopy, and choledochoscopy allows a minimally invasive approach to the treatment of Mirizzi syndrome (1,3,5,8,18,25,28).
Discussion
Signs and symptoms can be varied in literature; therefore the diagnostic method should be trough image studies. Literature differs regarding the use of laparoscopic cholecystectomy due to complications, however the majority indicates that the use of this procedure in Mirizzi syndrome type I is suitable and will have a slight margin of complications.
The open surgery is generally accepted as a way to improve the safety of any surgery because it allows the touch of a surgeon’s hand, especially if the inflammation is severe. However, many studies have found association between these procedures with an increase in long-term morbidity.
ERCP, despite being an invasive procedure, it is the method to diagnose Mirizzi syndrome, clean the common bile duct, and define any aberrant anatomy. When combined with duodenoscopy, laparoscopy, and choledochoscopy allows a minimally invasive approach for treatment.
There are different points of view about the specific moment for the diagnosis, since the signs and symptoms of the patient are not always specific, and this is the reason why making a preoperative diagnosis with certainty can be done in only 8–62.5% of patients. If the preoperative diagnosis is not made, intraoperative recognition and proper management is essential.
Carcinoma of the gallbladder is rare, but is the most common malignancy of the digestive tract, due to the chronic gallbladder inflammation; most commonly to gallstone disease with continuous mechanical gallbladder mucosal damage superimposed by biliary stasis (30). It has also been proposed that these same factors are responsible for the development of a porcelain gallbladder (31). A coexistent GBCa with Mirizzi syndrome is common. Redaelli et al. (30) in a retrospective study were reviewed 18 cases of Mirizzi syndrome and 5 of these patients (27.8%) had coincidental GBCa detected.
Conclusions
Mirizzi syndrome is a rare complication of biliary lithiasic worldwide with a nonspecific clinical picture in which approximately 1/3 of the cases develop asymptomatically. Although signs and symptoms are not clearly specific, the main clinical manifestation is jaundice with a predominantly obstructive pattern, and right upper quadrant pain; while hepatomegaly only occurs in a small amount of patients. An early and accurate diagnosis has a great impact on the management and prevention of future complications.
It is important to exclude the most common causes of bile duct obstruction of benign or malignant character. In most patients, making a preoperative diagnosis with certainty can be difficult initially because this condition can be confused with choledocholithiasis and cholangitis.
Despite the low sensitivity of USG, it is the best initial diagnostic study because of its low cost and accessibility. Cholangiopancreatography magnetic resonance is a noninvasive study with better specificity and sensitivity, although ERCP is still the best method for these patients.
Cholecystectomy is the treatment of choice in patients with Mirizzi syndrome, in which the procedure is done depending on the subtype of the syndrome. Much of the literature states that laparoscopic cholecystectomy is contraindicated in Mirizzi syndrome because it places the patient at high risk due to the adhesions and inflammatory tissue in the Calot triangle, if dissection is attempted may cause unnecessary injury to the bile duct. Meanwhile other surgeons claim that the laparoscopic technique is feasible, although technically challenging. As a consequence laparoscopic cholecystectomy for Mirizzi syndrome currently cannot be recommended as a standard procedure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abou-Saif A, Al-Kawas FH. Complications of gallstone disease: Mirizzi syndrome, cholecystocholedochal fistula, and gallstone ileus. Am J Gastroenterol 2002;97:249-54. [Crossref] [PubMed]
- Beltrán MA. Mirizzi syndrome: history, current knowledge and proposal of a simplified classification. World J Gastroenterol 2012;18:4639-50. [Crossref] [PubMed]
- Rayapudi K, Gholami P, Olyaee M. Mirizzi syndrome with endoscopic ultrasound image. Case Rep Gastroenterol 2013;7:202-7. [Crossref] [PubMed]
- Zaliekas J, Munson JL. Complications of gallstones: the Mirizzi syndrome, gallstone ileus, gallstone pancreatitis, complications of "lost" gallstones. Surg Clin North Am 2008;88:1345-68, x. [Crossref] [PubMed]
- Kelly MD. Acute mirizzi syndrome. JSLS 2009;13:104-9. [PubMed]
- Desai DC, Smink RD Jr. Mirizzi syndrome type II: is laparoscopic cholecystectomy justified? JSLS 1997;1:237-9. [PubMed]
- Wichmann C, Wildi S, Clavien PA. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg 2008;32:2244-5. [Crossref] [PubMed]
- Gomez D, Rahman SH, Toogood GJ, et al. Mirizzi's syndrome--results from a large western experience. HPB (Oxford) 2006;8:474-9. [Crossref] [PubMed]
- Safioleas M, Stamatakos M, Safioleas P, et al. Mirizzi Syndrome: an unexpected problem of cholelithiasis. Our experience with 27 cases. Int Semin Surg Oncol 2008;5:12. [Crossref] [PubMed]
- Lai EC, Lau WY. Mirizzi syndrome: history, present and future development. ANZ J Surg 2006;76:251-7. [Crossref] [PubMed]
- Chan CY, Liau KH, Ho CK, et al. Mirizzi syndrome: a diagnostic and operative challenge. Surgeon 2003;1:273-8. [Crossref] [PubMed]
- Erben Y, Benavente-Chenhalls LA, Donohue JM, et al. Diagnosis and treatment of Mirizzi syndrome: 23-year Mayo Clinic experience. J Am Coll Surg 2011;213:114-9; discussion 120-1. [Crossref] [PubMed]
- Kwon AH, Inui H. Preoperative diagnosis and efficacy of laparoscopic procedures in the treatment of Mirizzi syndrome. J Am Coll Surg 2007;204:409-15. [Crossref] [PubMed]
- Xu XQ, Hong T, Li BL, et al. Mirizzi syndrome: our experience with 27 cases in PUMC Hospital. Chin Med Sci J 2013;28:172-7. [Crossref] [PubMed]
- Tsuyuguchi T, Sakai Y, Sugiyama H, et al. Long-term follow-up after peroral cholangioscopy-directed lithotripsy in patients with difficult bile duct stones, including Mirizzi syndrome: an analysis of risk factors predicting stone recurrence. Surg Endosc 2011;25:2179-85. [Crossref] [PubMed]
- Jung CW, Min BW, Song TJ, et al. Mirizzi syndrome in an anomalous cystic duct: a case report. World J Gastroenterol 2007;13:5527-9. [Crossref] [PubMed]
- Fontes PR, Teixeira UF, Waechter FL, et al. Mirizzi syndrome in association with serum CA 19-9 greater than 20.000U/mL: is it possible? Arq Bras Cir Dig 2012;25:69-70. [Crossref] [PubMed]
- Antoniou SA, Antoniou GA, Makridis C. Laparoscopic treatment of Mirizzi syndrome: a systematic review. Surg Endosc 2010;24:33-9. [Crossref] [PubMed]
- Roesch-Dietlen F, Pérez-Morales AG, Martínez-Fernández S, et al. Mirizzi syndrome: experience at Spanish Hospital of Veracruz. Cir Cir 2013;81:232-6. [PubMed]
- Gibor U, Perry ZH, Netz U, et al. CA 19-9 in the presence of obstructive jaundice due to Mirizzi syndrome. Isr Med Assoc J 2015;17:60-1. [PubMed]
- Beltran MA, Csendes A, Cruces KS. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg 2008;32:2237-43. [Crossref] [PubMed]
- Horio T, Ogata S, Sugiura Y, et al. Cholecystic adenosquamous carcinoma mimicking Mirizzi syndrome. Can J Surg 2009;52:E71-2. [PubMed]
- Yonetci N, Kutluana U, Yilmaz M, et al. The incidence of Mirizzi syndrome in patients undergoing endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int 2008;7:520-4. [PubMed]
- Chatzoulis G, Kaltsas A, Danilidis L, et al. Mirizzi syndrome type IV associated with cholecystocolic fistula: a very rare condition--report of a case. BMC Surg 2007;7:6. [Crossref] [PubMed]
- Yeh CN, Jan YY, Chen MF. Laparoscopic treatment for Mirizzi syndrome. Surg Endosc 2003;17:1573-8. [Crossref] [PubMed]
- Palacios-Martínez D, Gutiérrez López M, Gordillo López FJ. Síndrome de Mirizzi, una causa infrecuente de ictericia obstructiva. Semergen 2011;37:167-9. [Crossref]
- Sanchez M, Gomes H, Marcus EN. Elevated CA 19-9 levels in a patient with Mirizzi syndrome: case report. South Med J 2006;99:160-3. [Crossref] [PubMed]
- Li B, Li X, Zhou WC, et al. Effect of endoscopic retrograde cholangiopancreatography combined with laparoscopy and choledochoscopy on the treatment of Mirizzi syndrome. Chin Med J (Engl) 2013;126:3515-8. [PubMed]
- Safioleas M, Stamatakos M, Revenas C, et al. An alternative surgical approach to a difficult case of Mirizzi syndrome: a case report and review of the literature. World J Gastroenterol 2006;12:5579-81. [Crossref] [PubMed]
- Redaelli CA, Büchler MW, Schilling MK, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery 1997;121:58-63. [Crossref] [PubMed]
- Nishimura A, Shirai Y, Hatakeyama K. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery 1999;126:587-8. [Crossref] [PubMed]


