Setting up a robotic hepatectomy program: a Western-European experience and perspective
Introduction
An increasing proportion of gastrointestinal procedures are performed minimally invasive. Nowadays, even complex procedures such as pancreatic and liver resections are done in a minimally invasive way (1-3). While in both pancreatic and liver surgery randomized clinical trials on patient benefit are underway, extensive non-randomized studies have already compared open with laparoscopic liver resection. Compared to open surgery, the laparoscopic approach has been associated with similar oncologic outcomes, shorter hospital stay and less blood loss, most evidently in minor liver resections (4-8).
Consequently, since the first conventional laparoscopic liver resection in 1992 (9), this technique has been gradually adopted by more and more predominantly very large hospitals. Surprisingly, however, the percentage of liver resections performed laparoscopically on a national health care level in many countries lags far behind that of other gastro-intestinal (e.g., colorectal) procedures. For instance, in the Netherlands in 2014 only 11% of liver resections were performed laparoscopically (10). Slow adoption of minimally invasive liver surgery may be due to the more complex anatomy of the liver, a highly vascularized solid organ, and the fact that many dedicated hepatobiliary- and HPB-surgeons are still “open” surgeons. The disadvantages of conventional laparoscopy (such as straight instruments with a 1-dimensional working axis and troublesome optics) are, therefore, most pronounced in liver surgery.
The use of a robotic surgical system can resolve these downsides of conventional laparoscopy. The view of the robotic system is 3-dimensional and instruments are wristed, with a range of motion greater than the human wrist. Robotic instrumentation may thus facilitate, for instance, curved parenchymal transection lines or dissection at the liver hilum. An additional advantage is less surgeon fatigue, especially in longer procedures (11,12). More than 400 robotic liver resections have recently been described in the literature [(13), reviewed in (14)], showing that robotic liver resection is safe and feasible, and may especially be of clinical advantage in smaller, ill-located partial hepatectomies.
In this review, we present our initial robotic hepatectomy case series and discuss the steps of setting up a robotic hepatectomy program against the background of the University Medical Center (UMC) Utrecht experience.
Initial experience with robotic hepatectomy at UMC Utrecht
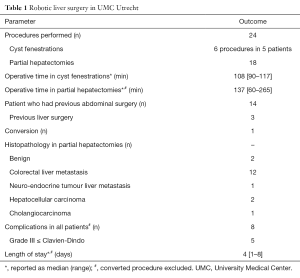
Since the start of the robotic hepatectomy program in August 2014, the hepato-pancreato-biliary team at UMC Utrecht performed 24 robotic liver surgeries.
In summary, 5 patients underwent a total of 6 cyst fenestration procedures [in part, published (15)] and 18 patients underwent a partial hepatectomy (Tables 1-3). All procedures were fully laparoscopic-robotic. Fourteen patients had had previous abdominal surgery, including 3 who had undergone liver surgery before. In the partial hepatectomies, a total of 21 resections were performed in 17 patients and 1 procedure was converted. The majority of the resections were performed for colorectal liver metastasis. The median operative time for our robotic hepatectomies was 137 minutes. Four patients had a grade III complication and one patient had a grade IV complication (Clavien-Dindo). The patient who had a grade IV complication suffered from a pulmonary embolism postoperatively and was admitted to a medium care unit for 2 days (16). We observed no grade V complications in our patients. In two of the patients with malignant disease, the surgical margin was positive (defined as tumour cells <1 mm distance to resection surface). Median length of hospital stay was 4 (range, 1–8) days. All patients visited our outpatient clinic after discharge: all had fully recovered and there were no wound healing problems.
Full table
Full table

Full table
Prerequisites for a robotic hepatectomy program
Several conditions must be met prior to starting the program. First and foremost, as anywhere in surgery, a successful program is a team effort. The team comprises the surgeons, as well as anaesthesiology, OR staff, and robotic support staff. At UMC Utrecht, we started out with two of the three HPB-surgeons performing each procedure, with one surgeon at the console for the first ten procedures and the other at the tableside, and switch for the next ten. In this way, experience is built by the team while the learning curve is steep enough for the individual.
Second, equipment and available expertise are needed. UMC Utrecht has one da Vinci robotic system (currently, the Si) in operation since 2000 that has mainly been used in urology and gastro-intestinal oncology surgery. Thus, at the time of starting the hepatectomy program, there was wide experience available within our department with robotic oesophagectomies, thyroidectomies, and distal pancreatectomies. While the HPB surgeons already had some experience performing robotic distal pancreatectomies (about 20 procedures performed by two surgeons), additional support was available from our upper-GI team and robotic physician assistant. In addition, the HPB surgeons all had extensive previous exposure in general surgical laparoscopy as well as limited experience with laparoscopic liver resection (mainly left lateral resections; around ten procedures performed per surgeon).
Third, proctoring is considered a crucial step in starting a program. Expertise worldwide in robotic liver resection, however, is sparse and still concentrated in a few hospitals. Therefore, in addition to official da Vinci console training (Paris, France), our team spent two multiple-day visits on case observations with Dr. Yuman Fong (Memorial Sloan-Kettering Cancer, New York; currently, City of Hope Medical Center, Duarte, CA, United States).
Patient selection
Patient characteristics
Indications for liver resection were set in our multidisciplinary HPB-tumour board meeting. Individual patients were next selected for robotic hepatectomy by the staff HPB surgeons based mainly on lesion location (i.e., lesions suitable for wedge, one- or two-segment resection without need for dissection at the hilum). Certain specific patient characteristics were specifically taken into account. First, body mass index (BMI). BMI of patients in our series BMI ranged from 18–33 kg/m2. Currently, there is no consensus on ‘ideal’ BMI for robotic liver surgery. Though, in extreme obesity or in patients with a very low BMI, it can be difficult to obtain enough working space and have adequate exposure. Furthermore, we excluded patients who had had very extensive abdominal surgery (e.g., complicated gastro-intestinal procedures) and severe comorbidities, such as patients with abnormal coagulation or conditions that precluded the patient from lying in anti-Trendelenburg. Patients who had undergone liver surgery were not excluded, provided previous resection was in the contralateral hemi liver. In our initial series, we performed two re-do robotic liver resections for colorectal metastases: a segment-2/3 metastasectomy after previous right trisectionectomy and a segment-2 metastasectomy after previous right hepatectomy.
Resection type
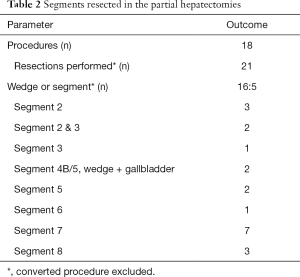
Following the 2008 Louisville Statement, and in the 2014 meeting in Morioka, Japan, it is recommended that surgeons implementing a laparoscopic liver resection program start with minor resections (defined as two or less segments) of segment 2, 3, 4B, 5 and 6. In the statement, resections of the posterosuperior segments (1, 7, 8, 4A) are considered ‘major resections’ and were not accepted as standard of care (17,18).
We started our robotic hepatectomy program according to these recommendations and first performed resections of the anterior and inferior segments. Alongside, robotic skill was further built with liver cyst fenestrations (15) and robotic distal pancreatectomies. However, due to the aforementioned benefits of the robotic system and based on first experience, we expected resections of the posterosuperior section to be technically less challenging robotically than with conventional laparoscopy. Hence, we successfully started early in the program with resections of segment 7 or 8.
Type of lesion
We performed the majority (12 out of 18 patients) of our resections for colorectal liver metastasis (median lesion size 19 mm, range 9–57 mm), since these lesions are common in our practice and often easy to locate by ultrasound, or by vision when subcapsular.
Other resected lesions included adenomas, neuro-endocrine liver metastases, intrahepatic cholangiocarcinoma, and hepatocellular carcinoma (HCC). One of two patients we resected for HCC was converted. This concerned a large (31 mm) HCC in segment 5 without clinical or radiological signs of liver cirrhosis or other parenchymal disease, which was deemed suitable for a wedge resection of segment 4B–5 along with the gallbladder. However, during surgery, the liver parenchyma appeared fibrotic and the lesion could not be properly delineated making it unclear if a safe oncologic margin could be obtained. The procedure was converted to a laparotomy, which resulted in a resection with a tumour free surgical margin. A cirrhotic or fibrotic liver can cause difficulties in parenchymal transection. Hence, although successful robotic resection for HCC has been reported in series from Asia (19), in our opinion, it would be preferable to not include HCC’s in an initial hepatectomy program, or to beware of a low threshold for conversion.
Surgical technique and robotic instruments
Patient positioning
In resections of left-sided and anterior segments, patients are placed in a 15 to 30-degrees reverse-Trendelenburg, French position. Four trocars are placed: one for the camera below the umbilicus, two robotic arms and a laparoscopic port for assistance. The robot is then docked over the patient’s head and the tableside assistant is positioned between the patient’s legs. For resections of posterosuperior segments, patients are placed in the left-lateral position (Figure 1). Four trocars are placed, and a fifth trocar for assistance where appropriate. The robot is docked over the patient’s head. All procedures were performed fully laparoscopic, no transthoracic trocars or hand ports were needed in any case.
Ultrasound and parenchymal transection
Intraoperative ultrasound is crucial in delineating oncological liver transection planes. We used a curved array 4-way laparoscopic transducer in our initial series (Hitachi Aloka Medical Inc., Wallingford, CT, USA). This laparoscopic transducer provided excellent imaging of the anterior liver segments, while imaging of segment-7 and segment-8 was felt to be less easy although adequate. Notably, a robotically controlled “drop-in” ultrasound transducer is on the market (Hitachi Aloka Medical Inc.) that may be of particular use in robotic liver surgery (13).
There are several techniques for parenchymal transection in robotic liver surgery (Table 4). It remains unclear, for both laparoscopic and robotic hepatectomy, which technique is best. In line with the absence of a clearly superior transection technique even in open surgery, it was recommended that surgeons should use the technique they are familiar with and that an individual assessment should be made per resection (18,20).

Full table
In open liver resection, the CUSA system is most frequently used in our center. This device is as of yet unavailable for the da Vinci Si surgical system. We mostly used a combination of the wristed Maryland bipolar and vessel sealer devices for parenchymal transection. The Endo GIA was used to control pedicles or larger branches of hepatic veins where deemed appropriate. A Pringle manoeuvre was applied in two patients.
TachoSil (Takeda Nederland b.v., Takeda, Zurich, Switzerland) was used on the resection surface where deemed appropriate. Given our initial experience with these novel transection techniques, a surgical drain was placed with low threshold (9 out of 24 patients). There were no postoperative bile leaks or hematomas in our initial patient series.
Combined procedures
In three patients we performed multiple segmentectomies in one procedure. In addition, two patients received a laparoscopic right hemicolectomy and sigmoidectomy, respectively, in the same procedure as their robotic hepatectomy. Standard laparoscopic colon resection was chosen in these cases, as a robotic hemicolectomy program is not set up in our hospital yet. Combining these procedures seems safe and feasible. This is in line with the largest case series on robotic hepatectomy to date, where 23 of the 70 patients underwent an associated surgical procedure (21). For the laparoscopic colectomies, the robot was undocked and the patient repositioned and redraped. For one of the multi-segmentectomies, we performed right-posterior resection first and then repositioned and redocked the robot to perform left sided resection. Potentially, the da Vinci Xi may overcome the inefficiency of re-docking in such cases as it permits multi-quadrant surgery.
Anaesthesia & perioperative care
All patients were operated under general anaesthesia with the first ten patients receiving an epidural catheter for analgesia. However, we omitted epidural anaesthesia after our 10th successful robotic hepatectomy and switched to patient-controlled analgesia where appropriate. Central venous pressure measurement, nasogastric tube placement and avoidance of excessive fluid administration were standard per-operative procedures per our liver resection protocol.
Postoperative care was according to the UMC Utrecht enhanced recovery after surgery (ERAS) protocol. Literature on ERAS specific for robotic hepatectomy is lacking. However, studies comparing ERAS versus traditional care for laparoscopic hepatectomy show that ERAS is safe and feasible and associated with less postoperative complications and shorter hospital stay. Therefore, we used this protocol for our patients who underwent robotic hepatectomy (22).
Evaluation and expansion of the program
The learning curve
Conventional laparoscopic liver resection may have a learning curve of up to 60 resections (23). For minor laparoscopic liver resections alone, learning curves have been reported ranging from 22 to 35 resections. Major laparoscopic have longer reported learning curves: 45 to 60 cases (24-26). Data on learning curves in robotic hepatectomy are not available. In would be interesting in the future to see if robotic hepatectomy has a steeper learning curve than laparoscopic hepatectomy. As for pancreatic resection for example: in pancreatic resection, regarded a highly complex procedure, a comparison has been made between learning curves in conventional laparoscopic resections and robotic resections. A significant shorter learning curve is shown for the robotic resections (27).
Evaluation and expansion
Our initial experience with robotic hepatectomy shows that this technique is easily adopted, allows for even fully laparoscopic, parenchyma-sparing resections of ill-located liver lesions, and is associated with low morbidity and fast recovery. The further expansion of our program will include training of an additional HPB-surgeon, volume expansion for the minor resections, dissemination of the technique in several other Dutch tertiary care hospitals to enable outcomes research, and emulation of the program with right and left hepatectomies in the near future.
Cost & healthcare context
As anywhere else, the use of robotics in surgery is under scrutiny. Downsides of the robotic system frequently mentioned by media and health care providers focussing on pure laparoscopic surgery, are the presumed higher costs and lack of evidence. Longer operative time and expensive equipment are two of the major reasons robotic surgery is expensive currently. Still, the potential shorter hospital stay and the fact that, theoretically, a larger proportion of liver resections can be performed minimally invasive, may compensate for this alongside the reduction in cost that may come from the introduction of competing robotic platforms in the near future. A recent study, comparing robotic with open liver resection, showed no difference in costs between these two techniques (28). Moreover, robotic sealing devices like the vessel sealer (Table 4) may be relatively less expensive than multiple laparoscopic stapler loads.
Health insurance in the Netherlands is mandatory and comprises a “hybrid” healthcare system where insurance companies are private (most not-for-profit branches of larger comprehensive, for-profit insurers), while overall health care expenditure and pricing is under strong government control. Within this context, there is no additional reimbursement (yet) for robotic gastro-intestinal surgical procedures. The university hospitals, however, have a responsibility and funding for tertiary care as well as research and innovation. The robotic program of UMC Utrecht is, therefore, partly funded via these academic resources.
Conclusions
In conclusion, robotic liver surgery can be safely and relatively easy implemented, provided that the start-up of the program is well coordinated. Due to the earlier mentioned benefits, indications for robotic liver resections can potentially be expanded using a robotic surgical system. In our opinion, the widespread introduction of minimally invasive surgery in liver resection will be most likely through robotic surgery.
Acknowledgements
The authors like to thank Prof. Richard van Hillegersberg and Dr. I. Quintus Molenaar for support during the set-up of our program.
Funding: Dr. J Hagendoorn was supported in part by KWF Personal Investigator Grant UU2014-6904.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ocuin LM, Tsung A. Minimally Invasive Hepatic Surgery. Surg Clin North Am 2016;96:299-313. [Crossref] [PubMed]
- Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9. [PubMed]
- de Rooij T, Klompmaker S, Abu Hilal M, et al. Laparoscopic pancreatic surgery for benign and malignant disease. Nat Rev Gastroenterol Hepatol 2016;13:227-38. [Crossref] [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-56. [Crossref] [PubMed]
- Parks KR, Kuo YH, Davis JM, et al. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB (Oxford) 2014;16:109-18. [Crossref] [PubMed]
- Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 2016;103:871-80. [Crossref] [PubMed]
- Alkhalili E, Berber E. Laparoscopic liver resection for malignancy: a review of the literature. World J Gastroenterol 2014;20:13599-606. [Crossref] [PubMed]
- Gagner M, Rogula T, Selzer D. Laparoscopic liver resection: benefits and controversies. Surg Clin North Am 2004;84:451-62. [Crossref] [PubMed]
- DICA. Rapportage 2014. Available online: http://www.clinicalaudit.nl/jaarrapportage/2014/dhba.html
- Diana M, Marescaux J. Robotic surgery. Br J Surg 2015;102:e15-28. [Crossref] [PubMed]
- Leung U, Fong Y. Robotic liver surgery. Hepatobiliary Surg Nutr 2014;3:288-94. [PubMed]
- Kingham TP, Leung U, Kuk D, et al. Robotic Liver Resection: A Case-Matched Comparison. World J Surg 2016;40:1422-8. [Crossref] [PubMed]
- Nota CL, Rinkes IH, Molenaar IQ, et al. Robot-assisted laparoscopic liver resection: a systematic review and pooled analysis of minor and major hepatectomies. HPB (Oxford) 2016;18:113-20. [Crossref] [PubMed]
- Nota CL, Molenaar IQ, Borel Rinkes IH, et al. Robot-assisted Laparoscopic Fenestration of Giant Hepatic Cysts. Surg Laparosc Endosc Percutan Tech 2015;25:e163-5. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 2013;205:697-702. [Crossref] [PubMed]
- Otsuka Y, Kaneko H, Cleary SP, et al. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2015;22:363-70. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery 2011;149:29-39. [Crossref] [PubMed]
- Ni TG, Yang HT, Zhang H, et al. Enhanced recovery after surgery programs in patients undergoing hepatectomy: A meta-analysis. World J Gastroenterol 2015;21:9209-16. [Crossref] [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [Crossref] [PubMed]
- Lin CW, Tsai TJ, Cheng TY, et al. The learning curve of laparoscopic liver resection after the Louisville statement 2008: Will it be more effective and smooth? Surg Endosc 2016;30:2895-903. [Crossref] [PubMed]
- Lee W, Woo JW, Lee JK, et al. Comparison of Learning Curves for Major and Minor Laparoscopic Liver Resection. J Laparoendosc Adv Surg Tech A 2016;26:457-64. [Crossref] [PubMed]
- Brown KM, Geller DA. What is the Learning Curve for Laparoscopic Major Hepatectomy? J Gastrointest Surg 2016;20:1065-71. [Crossref] [PubMed]
- Zeh HJ 3rd, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg 2011;45:323-40. [Crossref] [PubMed]
- Sham JG, Richards MK, Seo YD, et al. Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg 2016;10:307-13. [Crossref] [PubMed]


