Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common and the third most lethal cancer in China, accounting for 85–90% of primary liver malignancy (1). It is particularly prevalent in China because of the high prevalence of chronic hepatitis B infection. Since the 2011 version of consensus-based clinical practice guidelines published in China, new information has emerged that warrants a revised version to optimize the management of HCC. Herein, we summarize the recommendations on the surveillance, diagnosis and treatment algorithm in the 2017 updated guideline conducted by a multidisciplinary group of Chinese experts including liver surgeons, hepatic oncologists, radiologists, and pathologists. In particular, we make comparisons with established guidelines by the Japan society of Hepatology (JSH), the Asian-Pacific Association for the Study of the Liver (APASL), the American Association for the Study of Liver Diseases (AASLD), National Comprehensive Cancer Network (NCCN), European Association for the Study of the Liver-European Organization for Research and Treatment of Cancer (EASL- EORTC) (2-7).
Surveillance and diagnostic algorithm
Identical to the previous 2011 version, patients with chronic liver diseases and/or cirrhosis of any etiology are deemed as high-risk patients and should undergo AFP and B ultrasonography (US) every 6 months for surveillance. Besides AFP, a protein induced by vitamin K absence or antagonist-II (PIVKA-II) and AFP lectin fraction (AFP-L3) measurements are recommended by the JSH to increase sensitivity. Although the 2010 AASLD guideline preferred US alone, the 2017 updated American version suggests US with or without AFP for surveillance (4). One reason may lie in the fact that US + AFP group tended to have improved curative treatment rates and prolonged overall survival compared with US alone group, although no statistical significances were observed (8). In addition to high-risk population, the JSH further defined patients with HBV/HCV-related liver cirrhosis as super-high-risk population. For these super-high-risk patients, periodic imaging screening by US every 3–4 months and dynamic multidetector computed tomography (CT)/magnetic resonance imaging (MRI)/gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced MRI (Gd-EOB-DTPA-MRI) every 6–12 months is opposed. As no difference was detected between 3- and 6-month surveillance, our current guidelines insist on 6-month surveillance with regard to the cost-effectiveness (9,10).
Major changes have been made to the diagnostic criteria in the new version, as shown in Figure 1. Besides dynamic CT and MRI, Gd-EOB-DTPA-MRI and contrast-enhanced ultrasound (CEUS) are added to the diagnostic imaging tests, which have been established as diagnostic strategies by the JSH since 2010 (6). In addition to typical HCC hallmark presenting with hypervascularity in the arterial phase and washout in the portal venous and/or delayed phases, Gd-EOB-DTPA-MRI with demonstration of hypointensity in the hepatobiliary phase has shown superiority insensitivity particularly for detection of HCC ≤1 cm in diameter (11-13). It is noteworthy that hypointensity relative to the liver may otherwise reflect hyper enhancement of the liver parenchyma rather than de-enhancement of the liver parenchyma (pseudo-washout), leading to a decreased specificity for HCC diagnosis (14,15). With this regard, the AASLD and EASL guidelines adopt only dynamic CT/MRI as diagnostic modalities to avoid over diagnosis. Nevertheless, in most Asian countries such as China, Japan and Korea, which have the highest prevalence of HCCs globally, it is appropriate to recommend Gd-EOB-DTPA-MRI with high sensitivity and reasonably high specificity as a diagnostic method (6,16). Moreover, it has been demonstrated by a recent study that evaluation of early-stage HCCs by Gd-EOB-DTPA-MRI increased overall survival by detection of additional lesions (17). Sonazoid CEUS showing hypervascularity and/or a Kuppfer defect has also been used to diagnose HCC (18). On one hand, CEUS provides superior sensitivity for detecting arterial enhancement without nephrotoxicity and ionizing radiation (19). On the other hand, CEUS has a difficulty in discriminating between intrahepatic cholangiocarcinoma (ICC) and HCC since it is purely intravascular (20). Therefore, the AASLD and EASL committees removed CEUS from their guidelines in part to avoid false positive HCC diagnosis in patients with ICC. But according to recent studies, a wash-out time longer than 55 s is adequate to differentiate HCC from non-HCC malignancies, qualifying the addition of CEUS to the diagnostic modalities (21,22). Another change resides in the elimination of AFP as a confirmatory test in nodules of 1–2 cm in diameter, which becomes consistent with the recommendation worldwide. The diagnostic criteria of HCC in the newest Chinese guideline are as follows: for patients with chronic hepatitis B/C or cirrhosis of any etiology, nodules >2 cm in diameter can be diagnosed with HCC based on the typical features on one imaging technique whereas nodules ≤2 cm in diameter need two typical imaging findings for diagnosis. Otherwise, biopsy is recommended in case of inconclusive diagnosis. It should be noted that, for clinically diagnosed HCC with typical radiological hallmarks, biopsy is not suggested. Comparisons of diagnostic strategies among the current Chinese HCC guidelines and JSH, APASLD, AASLD, NCCN, EASL recommendations are summarized in Table 1.
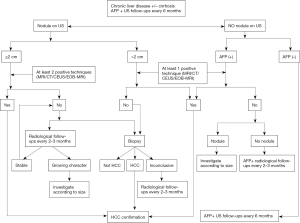
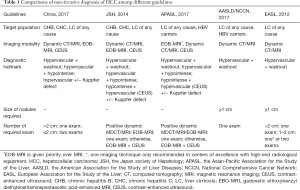
Full table
Although positron emission tomography-CT with [18F] fludeoxyglucose (FDG PET-CT) is not suggested as an initial diagnostic imaging method due to a limited sensitivity in detecting primary HCC, its application in evaluating regional lymphatic and distant metastasis is strongly recommended in the updated guideline (23). Moreover, it is encouraged for its use in selecting the tumor region most likely to yield diagnostic information for biopsy, guiding radiation therapy planning, detecting tumor recurrence in the presence of post-operative anatomical change or complex structures, evaluating the overall prognosis and monitoring the effect of targeted therapy (24-26). The pathological diagnosis of HCC samples is consistent with the 2015-updated standardization established by the Chinese Pathology Working Group for Liver Cancer (27). In brief, a novel 7-point baseline sample collection protocol is recommended in order to delineate tumor heterogeneity. Besides the routine description of microscopic characteristics including histological type, differentiation state, tumor growth patterns and adjacent liver diseases, microvascular invasion (MVI) is a newly added indicator for recurrence prediction. MVI, referred to the cancer cell nest in vessels lined with endothelial cells, is an independent prognostic marker for HCC (28). It is recommended to evaluate its presence in all tissue sections and grade as follows: M0: no MVI; M1 (low-risk): <5 MVIs and each ≤1 cm away from the adjacent liver tissues; and M2 (high-risk): >5 MVIs or at least one MVI >1 cm away from the adjacent liver tissues.
Staging and treatment algorithm
The previous 2011 version of HCC guideline in China endorsed TNM (UICC/AJCC, 2010) and Barcelona-Clínic Liver Cancer (BCLC, 2005) staging systems for prognostic prediction and treatment allocation (29,30). Based on updated evidences and clinical practices, a new staging system and treatment algorithm has been developed to be more comprehensible and suitable for use in China (Figure 2). The updated staging system in China focuses more on treatment allocation whereas TNM staging systems from the combined American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) or the Liver Cancer Study Group of Japan (LCSGJ) play more emphasis on postoperative prognosis (31,32). According to current recommendations, surgical resection remains the first-line treatment for single tumor or 2–3 nodules ≤3 cm in patients with well-preserved liver function and no evidence of extrahepatic lesions/vascular invasion. While radiofrequency ablation (RFA) was once recommended to be as effective as surgery for patients with a solitary HCC ≤5 cm, recent studies preferred surgery over RFA with regard to a better long-term prognosis (33-35). The 2017 AASLD guidelines also suggest that adults with Child’s A cirrhosis and resectable T1 or T2 HCC undergo resection over RFA (4). For unresectable solitary HCC ≤5 cm or 2–3 nodules ≤3 cm because of tumor location or impaired liver function, ablation therapy serves as a potentially curable treatment. The combination of ablation and transcatheter arterial chemoembolization (TACE) is more beneficial than monotherapy for unresectable solitary tumors measuring 3–7 cm in diameter (36,37). In consistence with the JSH guideline, resection in preference to TACE is recommended for patients with 2–3 tumors >3 cm, whereas TACE is the only option as suggested by the BCLC recommendations (38,39). In fact, resection is indicated for more progressed HCC in terms of tumor burden and for more diseased patients in terms of liver function in Asian countries (Table 2) (40). While normal bilirubin and portal pressure serve as a prerequisite for resection by the BCLC recommendations, slightly elevated bilirubin or portal hypertension is not a definite contradiction for surgical resection in Asia. In fact, hepatic venous pressure gradient (HVPG) is not a routine preoperative test in China. According to the current guideline, Child-Pugh score A, indocyanine green retention rate at 15 min (ICG-15) of 20–30% and residue/total liver volumetric CT of at least 40% (cirrhotic patients) or 30% (non-cirrhotic patients) are required for resection. It is acceptable to downstage initially oversize lesions by other local regional therapies (LRTs) or to optimize future liver remnant (FLR) by associating liver partition and portal vein ligation for staged hepatectomy (41) in patients with no or low-grade fibrosis (42,43). Minimally invasive surgical approaches including laparoscopic and robotic liver resection are recommended for appropriate patients at experienced centers. The previous 2011 guideline endorsed the Louisville criteria (solitary lesions ≤5 cm in diameter and located in the Couinaud segments II, III, IVb, V, VI) as the indications for laparoscopic liver resection (LLR) (44). It is now accepted that laparoscopic liver resections (LLRs) can be performed for large lesions generally ≤10 cm on the premise of no damage to the first and the second porta hepatis (45,46). Besides, major LLRs including hemihepatectomy, trisectionectomy and resection of the difficult posterior segments can also be tried by highly specialized surgeons. Up to date, it is still controversial about the definition of radical or curative resection for HCC. The revised definitions of radical resection in the current Chinese guideline incorporate the intraoperative and postoperative criteria rather than divide it into three grades. In detail, the intraoperative criteria include the three aspects: no gross tumor embolus in the hepatic vein, port vein, vena cava and bile duct; no extrahepatic spread; surgical margin ≥1 cm, or <1 cm but with R0 resection (no cancer cells found in surgical margin). The postoperative criteria for radical resection are fulfilled when the follow-up examinations two months after resection showed no evident tumor lesion by at least two of the three imaging tests including US, CT, MRI, and AFP decreased to the normal level for patients with preoperative increased AFP (the duration for AFP back to normal is longer than two months in very few patients).
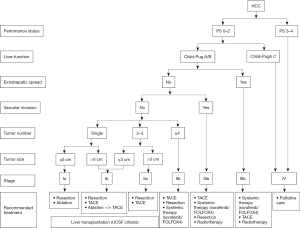
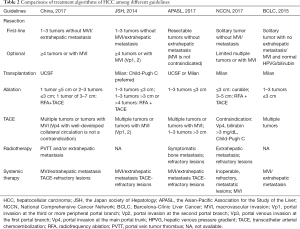
Full table
Liver transplantation is another radical therapy for selected patients with HCC. Despite the establishment of the Milan criteria (single tumor ≤5 cm or ≤3 nodules ≤3 cm in diameter without radiological evidence of vascular invasion or distant metastasis) as the golden candidate selection criteria for transplantation, many efforts has been made to benefit more patients by extending the indications in China (47-50). The current guideline suggests University of California San Francisco (UCSF) criteria (solitary tumor ≤6.5 cm or ≤3 nodules ≤4.5 cm plus total tumor diameter ≤8 cm without vascular invasion and extrahepatic metastasis) for wide use in China. Although the expansion of Milan criteria is not recommended by the EASL and JSH guidelines, recently updated AASLD guidelines suggest that patients beyond the Milan criteria can be candidate for transplantation after successful down-staging into the Milan criteria (51,52). In terms of liver function reserve, transplantation can be offered for Child-Pugh A/B cirrhotic patients in China and western centers, whereas liver graft is given priority to Child-Pugh C patients in Japan.
TACE is the mainstay of treatment for patients with more than three lesions and without vascular invasions/extrahepatic spread, namely patients at stage IIb, equivalent to BCLC B patients (53). Although only targeted therapy of sorafenib is indicated for stage IIIa patients with macrovascular invasion according to the BCLC recommendations, TACE is indicated for lesions with invasion at the second and the more peripheral portal branch in Japan, and even for lesions with portal vein tumor thrombus (PVTT) at the main trunk in China as long as collateral circulation is well-developed. While hepatic arterial infusion chemotherapy (HAIC) is commonly recommended for patients with portal invasion at the main portal trunk or at the first branch by the JSH guidelines, radiotherapies including transarterial radio embolization (TARE) and external radiation are more frequently used for this situation in China (54,55). For highly selective patients at stage IIb and IIIa, surgical resection is still indicated under the following situations: multiple lesions restrained in the same segment or the same lobe; lesions with PVTT in the same half liver which can be removed or resected. Adjuvant therapies including TACE and HAIC are recommended for patients with high risk of residue tumor and patients with PVTT at the main trunk respectively (56,57). Despite wide evaluation of interferon as an adjuvant agent, its efficacy is still controversial (58,59). Currently, only HCC patients with CHB are indicated for interferon in China. miR-26 was previously identified as a potential marker predicting the response to interferon (60). Its role as a predictor for adjuvant interferon is still under evaluation (NCT01681446).
Systemic therapies including sorafenib and FOLFOX4 chemotherapy are options of treatment for stage IIIb patients with extrahepatic metastasis and TACE refractory patients at stage IIb and IIIa. Although sorafenib is the only eligible systemic therapy in most countries, FOLFOX4 chemotherapy with a tendency towards improved overall survival has also been recommended as an option for Chinese patients with regard to the cost-effectiveness (61). Although TACE alone or in combination with radiotherapy showed a superior survival benefit over sorafenib for patients with macrovascular invasion and/or metastatic disease in some retrospective observational studies, it is impossible to make a recommendation for LRTs over systemic therapy in advanced HCC due to inadequate evidences (62-64). New targeted agents like regorafenib/lenvatinib and immune checkpoint PD-1/PD-L1 inhibitors are currently not available in China and may become promising treatment choices in the near future (65,66). Other systemic therapies including traditional Chinese medicine, immune-modulating therapy and differentiation-inducing therapy may also potentially benefit HCC patients. It is widely accepted that patients with end-stage diseases defined as tumors with Child-Pugh C cirrhosis beyond the transplantation threshold or a very poor performance status scoring 3–4 should receive palliative support. Despite the geographic differences in the treatment algorithm as shown in Table 2, it is agreed that a multidisciplinary team is needed to tailor specific therapies for HCC patients due to the complexity of treatment options.
Conclusions
The newest consensus-based Chinese guideline on the management of HCC mainly updates the diagnostic criteria and the treatment algorithm. Considering a particularly high prevalence of HCC in China, another two sensitive imaging techniques Gd-EOB-DTPA-MRI and CEUS are added to dynamic CT/MRI for early detection of HCC. Unlike the agreement on the treatment options for early HCC, therapeutic strategies for intermediate and advanced HCC vary greatly between western and eastern centers. Hepatectomy, transplantation and LRTs are indicated for more progressed HCC and for more diseased patients in China. Future efforts should be made to provide more evidences especially by RCTs for some consensus-based practices in the current guideline.
Acknowledgements
Funding: This study is funded by the National Natural Science Foundation of China (No. 81522036) and National Program for Special Support of Eminent Professionals.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 2014;3:458-68. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn R, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bruix J, Sherman M. American association for the study of liver diseases. management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339-64. [Crossref] [PubMed]
- Benson AB 3rd, D'Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:563-73. [Crossref] [PubMed]
- Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [Crossref] [PubMed]
- Andersson KL, Salomon JA, Goldie SJ, et al. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6:1418-24. [Crossref] [PubMed]
- Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987-97. [Crossref] [PubMed]
- Choi JY, Lee JM, Sirlin CB. CT. Radiology 2014;272:635-54. [Crossref] [PubMed]
- Lee DH, Lee JM, Baek JH, et al. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging in the detection of HCCs and allocation of transplant recipients on the basis of the Milan criteria and UNOS guidelines: correlation with histopathologic findings. Radiology 2015;274:149-60. [Crossref] [PubMed]
- Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015;275:97-109. [Crossref] [PubMed]
- Choi JY, Lee JM, Sirlin CB. CT. Radiology 2014;273:30-50. [Crossref] [PubMed]
- Joo I, Lee JM, Lee DH, et al. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol 2015;25:2859-68. [Crossref] [PubMed]
- Korean Liver Cancer Study G, National Cancer Center Korea. 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015;9:267-317. [PubMed]
- Kim HD, Lim YS, Han S, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;148:1371-82. [Crossref] [PubMed]
- Kudo M. Early hepatocellular carcinoma: definition and diagnosis. Liver Cancer 2013;2:69-72. [Crossref] [PubMed]
- Jang HJ, Kim TK, Burns PN, et al. CEUS: An essential component in a multimodality approach to small nodules in patients at high-risk for hepatocellular carcinoma. Eur J Radiol 2015;84:1623-35. [Crossref] [PubMed]
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010;51:2020-9. [Crossref] [PubMed]
- Wildner D, Bernatik T, Greis C, et al. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med 2015;36:132-9. [Crossref] [PubMed]
- de Sio I, Iadevaia MD, Vitale LM, et al. Optimized contrast-enhanced ultrasonography for characterization of focal liver lesions in cirrhosis: A single-center retrospective study. United European Gastroenterol J 2014;2:279-87. [Crossref] [PubMed]
- Lin CY, Chen JH, Liang JA, et al. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Radiol 2012;81:2417-22. [Crossref] [PubMed]
- Asman Y, Evenson AR, Even-Sapir E, et al. [18F] fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl 2015;21:572-80. [Crossref] [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]
- Hyun SH, Eo JS, Lee JW, et al. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona Clinic Liver Cancer stages 0 and A hepatocellular carcinomas: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging 2016;43:1638-45. [Crossref] [PubMed]
- Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279-87. [Crossref] [PubMed]
- Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325-39. [Crossref] [PubMed]
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol 2017. [Epub ahead of print]
- Kudo M, Kitano M, Sakurai T, et al. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the liver cancer study group of Japan. Dig Dis 2015;33:765-70. [Crossref] [PubMed]
- Liu PH, Hsu CY, Hsia CY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤2 cm in a Propensity Score Model. Ann Surg 2016;263:538-45. [Crossref] [PubMed]
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. [Crossref] [PubMed]
- Xu Q, Kobayashi S, Ye X, et al. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep 2014;4:7252. [Crossref] [PubMed]
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. [Crossref] [PubMed]
- Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116:5452-60. [Crossref] [PubMed]
- Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Ho MC, Hasegawa K, Chen XP, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014). Liver Cancer 2016;5:245-56. [Crossref] [PubMed]
- Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829-36. [Crossref] [PubMed]
- Chan AC, Poon RT, Chan C, et al. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg 2016;263:e14-6. [Crossref] [PubMed]
- D'Haese JG, Neumann J, Weniger M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol 2016;23:1335-43. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Levi Sandri GB, de Werra E, Masciana G, et al. Laparoscopic and robotic approach for hepatocellular carcinoma-state of the art. Hepatobiliary Surg Nutr 2016;5:478-84. [Crossref] [PubMed]
- Moris D, Vernadakis S. Laparoscopic hepatectomy for hepatocellular carcinoma: the opportunities, the challenges, and the limitations. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- Fan J, Yang GS, Fu ZR, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 2009;135:1403-12. [Crossref] [PubMed]
- Li J, Yan LN, Yang J, et al. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009;15:4170-6. [Crossref] [PubMed]
- Hołówko W, Wróblewski T, Wojtaszek M, et al. Transarterial chemoembolization prior to liver transplantation in patients with hepatocellular carcinoma. Ann Transplant 2015;20:764-8. [Crossref] [PubMed]
- Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl 2015;21:1142-52. [Crossref] [PubMed]
- Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. [Crossref] [PubMed]
- Hou JZ, Zeng ZC, Wang BL, et al. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Jpn J Clin Oncol 2016;46:357-62. [Crossref] [PubMed]
- Yang M, Fang Z, Yan Z, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol 2014;140:211-9. [Crossref] [PubMed]
- Ren ZG, Lin ZY, Xia JL, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol 2004;10:2791-4. [Crossref] [PubMed]
- Fan J, Zhou J, Wu ZQ, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2005;11:1215-9. [Crossref] [PubMed]
- Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg 2007;245:831-42. [Crossref] [PubMed]
- Mazzaferro V, Romito R, Schiavo M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006;44:1543-54. [Crossref] [PubMed]
- Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med 2009;361:1437-47. [Crossref] [PubMed]
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501-8. [Crossref] [PubMed]
- Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol 2015;26:320-9.e6. [Crossref] [PubMed]
- Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol 2014;14:84. [Crossref] [PubMed]
- Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol 2015;50:445-54. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Killock D. Immunotherapy: Nivolumab keeps HCC in check and opens avenues for checkmate. Nat Rev Clin Oncol 2017;14:392. [Crossref] [PubMed]


