International consensus guidelines on robotic pancreatic surgery in 2023
Highlight box
Key recommendations
• Robotic distal pancreatectomy is associated with less intraoperative blood loss, less length of hospital stay (LOH) compared to open surgery;
• Robotic spleen-preserving distal pancreatectomy is feasible and comparable to open and laparoscopic surgery;
• In experienced hands, robotic pancreatoduodenectomy is associated with lower estimated blood loss, and shorter LOH compared to open surgery;
• Robotic radical antegrade modular pancreatosplenectomy (RAMPS) is safe and feasible with comparable outcomes compared to open RAMPS;
• Robotic pancreatic enucleation can be applied efficiently in superficial benign tumors;
• Ergonomic factors, social factors, and personal factors such as the LOH should be evaluated instead of only focusing on the expense caused by surgery.
What is known and what is new?
• Previous expert consensus lacked the latest evidence-based medical evidence to address providing comprehensive guidance for the clinical practice of robotic pancreatic surgery (RPS);
• Our recommendations offer comprehensive advice on indications, safety, and efficacy of different types of RPS.
What is the implication, and what should change now?
• This consensus summarizes the latest RPS literature, bringing together the opinions and recommendations of 29 Eastern and Western experts on 19 clinical issues;
• Future research topics include randomized controlled studies of RPS versus open and laparoscopic surgery and how to improve short- and long-term outcomes of robotic pancreatectomy.
Introduction
The use of robotic surgery in pancreatic procedures such as distal pancreatectomy (DP), pancreatoduodenectomy (PD), central pancreatectomy (CP), total pancreatectomy (TP), pancreas tumor enucleation, Appleby operation, radical antegrade modular pancreatosplenectomy (RAMPS) and pancreatic surgery with vascular resection have increasingly been described over the last decades (1-7). In a previously published international guideline statement on robotic pancreatic surgery (RPS), a summary was provided on the relevant topics of RPS (8). The following Miami Guidelines on Minimally Invasive Pancreatic Surgery addressed some of these topics and demonstrated the safety and feasibility of RPS in some pancreatic procedures (9). However, many topics remained unclear due to the lack of data to support the current evidence (10,11), leaving the debate on the further applicability of robotic surgery. Ever since a variety of high-quality multicenter studies have been published investigating the application of RPS. As new evidence is available now, current literature needs to be reviewed to update current existing guidelines on RPS.
The evidence-based guidelines of the current project are developed by experts in pancreatic surgery worldwide. Evidence-based medicine methods including thorough literature reviews, evaluation of systematic reviews and meta-analyses, and adopting the GRADE methodology to assess the level of evidence and strength of recommendations were all carried out with the help of professional methodologists. Guidelines on RPS as well as relevant clinical questions and topics were subsequently created. We present this article in accordance with the CREDES reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-132/rc) (1).
Methods
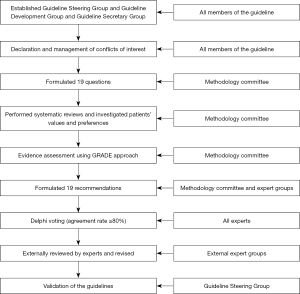
According to the World Health Organization (WHO) Handbook for Guideline Development, a Guideline Steering Group* of six experts, a Guideline Development Group of a multidisciplinary group of 22 experts, and a Guideline Secretary Group were established to develop the guidelines. The specific missions for each group were defined as follows:
Guideline Steering Group: (I) approve the use of PICOs (population, intervention, comparator, outcomes); (II) supervise the literature search and systematic reviews; (III) check the grade of the evidence; (IV) draft the final recommendations using a modified Delphi approach; (V) approve the publication of the guideline.
Guideline Development Group: (I) define the scope of the guideline and draft the PICOs; (II) grade the quality of the evidence; (III) draft preliminary recommendations; (IV) write the draft guideline; (V) publish and promote the guideline.
Guideline Secretary Group: (I) conduct systematic reviews and investigation of patients’ views and preferences; (II) provide methodological support.
After the approval of the clinical questions included in the current guideline, the following steps were conducted one by one (Figure 1):
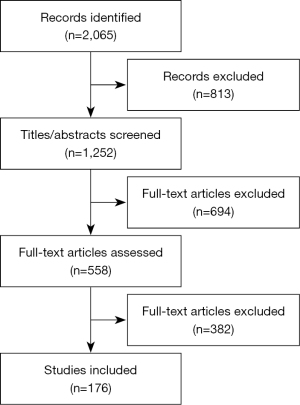
- Literature review: PubMed, Embase, the Cochrane Library, and three Chinese literature databases (CNKI, Wanfang, and CBM) from inception to October 2021 were used to perform the systematic literature review including published articles and reviews including conference abstracts (Figure 2).
- Summary of studies: a summary of the literature including evidence summary tables using the GRADE approach was performed for all included questions.
- Recommendations: the GRADE Grid method and Delphi vote were used to formulate the recommendations and establish consensus through three rounds of voting meetings. Only recommendations with more than 80% agreement among experts who attended the meeting were approved as a guideline.
- Validation: all recommendations were submitted to 20 external reviewers with enough minimally invasive hepato-pancreato-biliary (HPB) surgery experience who were not included in the guideline voting process. Disagreements on the not approved recommendations were discussed by the Guideline Steering Group.
The three online voting rounds were held on 04/12/2020, 30/11/2021, 25/01/2022. At each meeting, there were more than 20 attending experts. After the three meetings, the manuscript was finished and revised. All experts involved in the guideline project reviewed the drafting version, gave their comments, and eventually approved for the final publication.
Results
Question 1: What are the indications for a robotic DP (RDP)?
RDP is suitable for benign and malignant tumors located in the body-tail of the pancreas as well as large benign tumors and malignant tumors requiring associated vascular procedures.
Level of evidence: moderate. Grade 1B. Strong recommendation (expert agreement 95.0%, audience agreement 90.0%).
Comments: for benign and low-grade malignant tumors, RDP is superior in operation time, estimated blood loss, spleen preserving rate, and infection rate compared with open DP (ODP) (12,13). The Dutch LEOPARD randomized controlled trial (RCT) compared the perioperative outcomes of ODP with laparoscopic and robotic procedures and found a reduction in time to functional recovery in minimally invasive DP (MIDP) (14). For pancreatic ductal adenocarcinoma (PDAC), a retrospective pan-European study showed a comparable survival after MIDP and ODP, with improved R0 resection rate and contradictory lymph node retrieval (15). Subsequently, the international multicenter DIPLOMA RCT was conducted with the aim to demonstrate the non-inferiority of MIDP compared to ODP regarding oncological outcomes (16). Results are expected in 2023. Another recent meta-analysis concluded an improved R0 resection rate of RDP compared with laparoscopic DP (LDP), with no difference in harvested lymph nodes (17). RDP showed comparable disease-free survival (DFS) and overall survival (OS) with LDP in one study (18). Another study demonstrated a higher median OS of RDP compared with LDP and ODP in PDAC patients (19). No difference in long-term quality of life was seen after RDP and ODP (20).
Question 2: Is an RDP safe and feasible?
RDP is associated with less intraoperative blood loss, less length of hospital stay (LOH) compared to ODP.
Level of evidence: moderate. Grade 1B. Strong recommendation (expert agreement 96.0%, audience agreement 91.0%).
Comments: lower estimated blood loss, transfusion rate, postoperative mortality rate, and shorter length of stay were observed after RDP compared with ODP. RDP has a significantly higher R0 rate and lower conversion rate compared with LDP (6,14). No differences in operation time estimated blood loss, severe complications, lymph node harvested, and LOH were observed (21-23). No significant differences were found in operating time, lymph nodes harvested, R0 rate, spleen preservation rate, severe morbidity rate, and postoperative pancreatic fistula (POPF) rate between the two groups (24,25). Significant lower POPF rates were found after RDP in patients without visceral obesity (26). RCTs comparing RDP to LDP are lacking but needed.
Question 3: What is the learning curve for RDP?
Twenty procedures are considered sufficient to complete the learning curve for operating time in RDP.
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 85.0%, audience agreement 80.0%).
Comments: CUSUM method based on operation time was used to evaluate the learning curve of RDP. The first inflection points were 20 and 37 respectively in two studies (27,28). Prior experiences with robotic surgery may shorten the learning curve (27). Formal mentorship and skills curriculum of initial robotic surgeons can decrease the learning curve (29).
Question 4: Should robotic surgery be used for spleen-preserving DP?
Robotic spleen-preserving DP (R-SPDP) is feasible and comparable to open surgery. R-SPDP is feasible and comparable to laparoscopic surgery [laparoscopic spleen-preserving DP (L-SPDP)].
Level of evidence: moderate. Grade 1B. Strong recommendation (expert agreement 93.0%, audience agreement 91.5%).
Comments: a meta-analysis enrolling 11 studies between 2011 and 2020 compared the spleen preservation rate of R-SPDP and L-SPDP. Results indicated that R-SPDP was superior to L-SPDP regarding the failure of spleen preservation. Reduced conversion rates to open surgery, blood loss and LOH were observed. Meanwhile, operation time, B/C POPF, and other severe postoperative complications showed no significant differences (30). A recent multicenter cohort study also confirmed improved spleen preservation of RDP (25). The robotic Kimura and Warshaw procedures were both safe and feasible with no significant differences in complications between both groups (31). Compared with L-SPDP, R-SPDP has a higher spleen vascular preserving rate (32-34). Associated costs were higher in R-SPDP (35). One cohort study compared open spleen-preserving DP (O-SPDP) with minimally invasive spleen-preserving DP (MI-SPDP), which was composed of 10 laparoscopic and 13 robotic surgeries. Poorer long-term splenic vein patency rates occurred in the MI-SPDP group compared with O-SPDP. Further research is needed to clarify the difference between R-SPDP and O-SPDP (36).
Question 5: What are the indications for robotic CP (RCP)?
RCP is suitable for benign and borderline tumors in the neck and proximal body of the pancreas.
Level of evidence: moderate. Grade 1B. Strong recommendation (expert agreement 96.0%, audience agreement 92.0%).
Comments: for benign or low-grade malignant tumors located at the neck of the pancreas, compared with RDP, RCP showed less estimated blood loss, but was associated with higher POPF rate and LOH. No significant improvement in the postoperative pancreatic function was observed after RCP (37). Comparison of an end-to-end pancreatic anastomosis with a pancreaticojejunostomy (PJ) after RCP was performed by Wang et al. (38). End-to-end pancreatic anastomosis was associated with shorter operative time and reduced blood loss and equivalent postoperative endocrine or exocrine function but was related to a higher POPF rate (38). Large international studies are lacking but needed.
Question 6: Is an RCP safe and feasible?
RCP is considered as safe and efficient as open CP (OCP).
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 87.0%, audience agreement 82.0%).
Comments: blood loss is significantly less in RCP compared to OCP, but the operation time remains controversial (39,40). No differences in post-operative diabetes, POPF, and complication rates were observed between RCP and OCP (39). Additionally, RCP is considered to have low perioperative mortality but a high overall POPF rate (41). Minimally invasive CP is safe and effective in preserving endocrine and exocrine functions in treatment of benign or borderline tumors located at the neck or proximal body of the pancreas (42).
Question 7: What is the learning curve for RCP?
More than 20 consecutive cases are needed to surpass the safety proficiency learning curve.
Level of evidence: very low. Grade 2D. Weak recommendation (expert agreement 81.0%, audience agreement 80.0%).
Comments: two inflection points were observed after 12 and 44 cases of the RCP learning curve. Improvement in operation time and blood loss were achieved after the 44th case. There was no significant difference in perioperative complications between the learning and maturation learning curve phase (43).
Question 8: What are the indications for robotic PD (RPD)?
RPD is suitable for benign and malignant tumors of the pancreatic head and periampullary region as well as borderline resectable tumors of the pancreatic head requiring PD.
Level of evidence: moderate. Grade 1B. Strong recommendation (expert agreement 88.0%, audience agreement 85.0%).
Comments: compared with open PD (OPD), in experienced hands RPD is technically feasible showing no increase in surgical risk (44-52) as well as oncologically justifiable showing comparable survival outcomes for PDAC (53-56), primary non-ampullary duodenal adenocarcinoma (57), intraductal papillary mucinous neoplasms (IPMN) (58) and other low-grade malignant tumors (59). Histological outcomes also favor RPD (51,60). In high-volume centers, RPD with venous reconstruction is feasible (61,62). In patients who received neoadjuvant treatment, RPD is associated with shorter LOH, better lymphadenectomy, higher receipt of adjuvant chemotherapy, and similar OS (63). RPD is also associated with a lower operation time, anastomosis time, and conversion rate compared to laparoscopic PD (LPD) (64,65). Postoperative complications, including POPF, are comparable between both procedures (65,66). Obesity is an independent risk factor for the implementation of RPD (67). RPD is also safe and feasible in elderly patients (68). Structured implementation of RPD is suggested to achieve better outcomes (69). Formal RPD training in sufficient volume centers is feasible without a negative impact on clinical outcomes (70-72). Overall costs of RPD are higher compared to open PD (73,74). RCTs on RPD vs. open PD are lacking but clearly needed. In China the multicenter PORTAL trial is ongoing (75), in Europe the single center EUROPA RCT has recently been completed (76), and the international multicenter DIPLOMA-2 RCT (https://doi.org/10.1186/ISRCTN27483786) is ongoing.
Question 9: Is a robotic pancreaticoduodenectomy safe and feasible?
In experienced hands, RPD is associated with lower estimated blood loss, and shorter LOH compared to OPD.
Level of evidence: moderate. Grade 1B. Strong recommendation (expert agreement 90.0%, audience agreement 87.0%).
RPD attains similar R0 resection and a higher lymph node yield compared to OPD.
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 83.5%, audience agreement 81.0%).
Comments: in contrast with OPD, RPD showed slight favorable short-term outcomes according to several published studies (45,77-82). The available systematic reviews on RPD versus OPD, including a high amount of recently published studies, indicated that RPD was associated with lower rates of wound infections, and lower overall complications (45,77,79,81). In general, RPD can attain around 200 mL less blood loss and nearly 3 days shorter LOH compared to OPD, which potentially may contribute to enhanced functional recovery. Oncologically outcomes such as R0 resection rate and lymph node harvest rate are comparable between RPD and OPD (60,83-86). In several studies, RPD is associated with a higher number of harvested lymph nodes in experienced hands (60,83-85). RCTs focusing on the surgical and oncological outcomes of RPD as compared to OPD are needed and expected.
Question 10: What is the learning curve of RPD?
More than 50 consecutive cases are needed to surpass the RPD safety proficiency learning curve.
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 82.0%, audience agreement 80.0%).
Comments: the prior guideline study showed that 80 RPD cases are needed to get comparable operative times to OPD and 120 RPD cases to achieve optimal estimated blood loss and comparable 90-day mortality (87). Recent studies showed that more than 40 RPD cases were needed to achieve comparable perioperative outcomes to benchmark outcomes in low-risk patients (71,72,88). Generally, operative time is the most commonly used variable to evaluate the learning curve, but still not considered very efficient. The European Consortium on Minimally Invasive Pancreatic Surgery reported that over 60 RPD cases were needed for the safe implementation of RPD (89). However, the accurate definition of a proficiency learning curve in RPD is still lacking and should be defined in future studies.
Question 11: What is required to perform RPD with vascular resection and reconstruction?
Surgeons who have surpassed the RPD safety proficiency learning curve are potential to perform RPD with resection/reconstruction of portal vein/superior mesenteric vein (PV/SMV).
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 84.0%, audience agreement 85.0%).
Comments: RPD with vascular resection, especially with the PV/SMV is safe and feasible for surgeons who have surpassed the RPD proficiency learning curve (4,90). RPD-SMV/PV is associated with a longer operative time and a higher mean number of examined lymph nodes with the same rate of microscopic margin positivity (4). Compared to RPD alone, RPD with vascular resection showed comparable rates of pancreatic fistula, major morbidity, and mortality in selected patients (91). Additionally, it takes over 35 RPDs with vascular resection to achieve an improvement in surgical performance. In contrast to OPD with vascular resection, RPD with vascular resection showed no significant difference in 30-day morbidity and 90-day mortality, and 3-year survival rates (61).
Question 12: Is a robotic RAMPS safe and feasible for pancreatic cancer?
Robotic RAMPS is safe and feasible with comparable outcomes compared to open RAMPS.
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 90.0%, audience agreement 87.0%).
Comments: robotic surgery is an efficient approach for a RAMPS procedure and can be performed safely by surgeons who have surpassed their RDP proficiency learning curve (92-94). A robotic RAMPS procedure can be applied routinely and provides comparable DFS and OS compared to an open RAMPS (6,95). The proficiency learning curve of a robotic RAMPS can be achieved after 65 procedures (96). RCTs are lacking but needed to compared outcomes between the robotic and laparoscopic approaches.
Question 13: What are the indications for a robotic parenchyma-sparing procedure?
Robotic pancreatic enucleation can be applied efficiently in superficial benign tumors.
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 87.0%, audience agreement 88.0%).
Comments: robotic pancreatic enucleation is technically safe and feasible for benign pancreatic tumors (8,97-99). Robotic pancreatic enucleation shows less conversion to open surgery compared to laparoscopic pancreatic enucleation with comparable short-term outcomes (100). Besides, robotic pancreatic enucleation is associated with less trauma, faster wound recovery, less intraoperative blood loss, shorter LOH, shorter operative time, less overall complications with no differences in long-term outcomes (101-104). RCTs are lacking.
Question 14: How to assess the cost-effectiveness of RPS?
Ergonomic factors, social factors, and personal factors such as the LOH should be evaluated instead of only focusing on the expense caused by surgery.
Level of evidence: low. Grade 2C. Weak recommendation (expert agreement 91.0%, audience agreement 93.0%).
Comments: in a previous study, RDP showed similar short-term outcomes but no cost-effectiveness compared to LDP (105). Other studies indicated that RDP had similar cost-effectiveness and favorable short-term outcomes compared to LDP (106,107). Recent studies showed that RDP was cost-effective, taking into account the reduced LOH and faster functional recovery compared to ODP (108-111). In terms of overall costs and overall cost-effectiveness of RPS, the intraoperative cost is currently the only considered factor in the evaluation of the cost-effectiveness, however, other important factors such as social factors and personal factors should also be considered (112,113).
Question 15: Should staple versus another type of closure be used for stump closure in RDP?
Both stapler and non-stapler closure can be used in RDP (expert agreement 92.0%, audience agreement 89.0%).
Comments: during a DP, both staplers, as well as non-stapler closure, are used for pancreatic stump closure and no differences were observed between both methods in previous studies (114,115). In RDP, stapler and non-stapler closure are both technically safe and feasible (116,117). A recent study demonstrated that RDP with a reinforced stapler could potentially lead to reduced POPF rates (118). RCTs are lacking in this field.
Question 16: How to repair a main pancreatic duct (MPD) injury during robotic pancreatic enucleation?
The salvage pancreatectomy or pancreatic-enterostomy combined with an exemption should be applied for the injured MPD mainly caused by surgical trauma (expert agreement 86.0%, audience agreement 84.0%).
Comments: during pancreatic surgery, especially in pancreatic enucleation for a deep neoplasm, the MPD is considered to be at risk for injury (119,120). A salvage pancreatectomy or pancreatic-enterostomy combined with an exemption should be considered for a MPD injury caused by surgical trauma (121,122). In addition, it is considered to be safe to adequately drain the area of damage and suture the site of damage because large vessels are isolated from contact with the pancreatic juice. Surgeons should consider the preoperative surgical approach to reduce the potential risk of POPF caused by MPD injury.
Question 17: Which pancreatico-intestinal anastomosis should be used in RPD?
PJ or pancreaticogastrostomy (PG) are feasible to be applied by robot (expert agreement 87.0%, audience agreement 88.0%).
Comments: the unique advantages of the robotic surgical system, such as the 3D field of vision, can provide great benefits for pancreatic anastomosis (123). Various techniques could be used for pancreatoenterostomy anastomosis in RPD, and consistent with OPD, the most common pancreatic reconstruction method in RPD is a PJ anastomosis (8). POPF after pancreatic reconstruction remains a serious concern for surgeons (124,125). In a propensity score matched study comparing RPD and OPD with a modified Blumgart PJ anastomosis, Wang et al. (124) found no significant difference in the incidence of clinically relevant POPF (CR-POPF) between both groups. Menonna et al. indicated that the modification of the Blumgart PJ anastomosis is feasible in RPD. Additionally, PG anastomosis is also a common choice in pancreatic anastomosis. Although Giulianotti et al. (126,127) reported that the end-to-side PJ anastomosis could be performed if the MPD was more than 3 mm, and when the MPD was less than three mm, the POPF rate was high after PJ anastomosis, so a PG reconstruction was recommended. To date, there is no clear recommendation on the standard technique for pancreatic anastomosis, and no reliable evidence to support the use of PJ over PG (128). RCTs are lacking in this field.
Recently, various new methods of pancreatic anastomosis have been explored and showed good clinical results (129,130). Liu et al. (130) demonstrated that a single-layer pancreatojejunostomy is not inferior to a modified Blumgart anastomosis in RPD. Kiguchi et al. (131) proved that a novel wrapping double-mattress anastomosis in minimally invasive pancreaticoduodenectomy (MIPD) significantly reduced the POPF rate. Accordingly, the choice of a pancreatic anastomosis method is based on surgeons’ expertise and their discretion (132).
Question 18: What are the conversion rates of RPS to open surgery?
The conversion rate from a robotic procedure to open surgery is lower than the conversion rate from the laparoscopic approach to open surgery (expert agreement 96.0%, audience agreement 94.0%).
Comments: robotic surgical systems can increase the range of motion and reduce hand tremors (131) and may overcome some limitations of laparoscopic systems, such as the fulcrum effect caused by laparoscopic instruments. Most studies have concluded that the robotic approach has a lower conversion rate than the laparoscopic approach after completion of the learning curve (21,133-138). The Pittsburg group reported a steep decline in conversion rate after 20 RPD procedures (35% vs. 3.3%) (87). de Rooij et al. (136) showed that the conversion rate of RDP was 38% during the early phase of the learning curve, and decreased to 8% after the implementation of a training program. A systematic review and meta-analysis including 3,462 patients (1,025 robotic and 2,437 laparoscopic) demonstrated that RPD was associated with significantly lower conversion rates compared to LPD (135). Nassour et al. (134) compared the data of 235 patients who underwent LPD and 193 patients who underwent RPD and found RPD was associated with a lower conversion rate.
A systematic review and meta-analysis comparing the perioperative outcomes of RDP and LDP indicated that RDP reduces conversion rates without increasing operative time (21). In addition, Kamarajah et al. (138) analyzed 21 randomized studies including 3,112 patients (793 robotic and 2,319 laparoscopic) and showed that RDP was associated with significantly lower conversion rates compared to LDP.
Since converted patients have higher complication rates and worse oncological outcomes (139,140), the low conversion rate for the robotic approach may lead to faster recovery and permit earlier treatment with adjuvant therapy in patients with malignancies (135). Thus, careful selection of appropriate patients is essential when considering a robotic approach, and surgeons in their early learning curves of robotic surgery should be cautious, especially in patients at high risk for conversion (133).
Question 19: Can RPS be performed after neoadjuvant therapy (NAT)?
Robotic pancreatic resections are possible in patients who received neoadjuvant medical treatments (expert agreement 88.0%, audience agreement 91.0%).
Comments: in recent years, NAT has become a standard preoperative program for patients with borderline resectable and locally advanced pancreatic cancer due to its advantages in increasing R0 resection rate, screening patients with good response to adjuvant therapy, and improvements in prognosis (141,142). A recent study comparing the safety and oncological efficacy of RPD and OPD after neoadjuvant chemotherapy in pancreatic cancer found that after neoadjuvant chemotherapy, RPD is associated with similar mortality and a shorter length of stay, a higher proportion of adequate lymphadenectomy and a higher receipt of adjuvant chemotherapy (63). In another study, which compared robotic with ODP with celiac axis resection (DP-CAR) for locally advanced pancreatic body tumors, 27 of 28 pancreatic adenocarcinoma patients received neoadjuvant chemotherapy. The results showed that RDP-CAR was associated with a decreased operative time (316 vs. 476 min), reduced estimated blood loss (393 vs. 1,736 mL), and lower rates of blood transfusion (0% vs. 54%) (all P<0.05). Both approaches had high R0 resection rates (82% vs. 79%) with a median survival approaching 3 years (143). Krell et al. (144) used the data of the 2014–2018 American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) to evaluate NAT use for PDAC patients undergoing pancreatectomy and found that patients who received NAT were more likely to undergo vascular resections. AlMasri et al. (145) demonstrated that in high-volume centers, the robotic approach can be safely used in selected cases of technically challenging borderline-resectable pancreatic head cancers after neoadjuvant chemotherapy. Furthermore, NAT may be associated with a significant reduction in the rate of CR-POPF, which needs to be further validated in future prospective RCTs (144,146). RCTs are lacking in this field.
Conclusions
Above all, RPS is considered to be truly safe and feasible when performed by experienced hands in high-volume centers. Additionally, RPS may results in less blood loss and shorter LOH although RCTs are currently lacking. Besides, the long-term outcomes are not enough for the comprehensive evaluation up to date leading to oncological assessments being not prudent until now. Hence, the systematic evaluation for the RPS is expected to be continued in future.
For the implementation of RPS, knowledge on the learning curve and required hospital volume are essential. Further, the standard evaluation of RPS combined with the ergonomic factors, social factors and the quality of operation is suggested to be improved for the extensive evaluation of pancreatic surgery. High-quality studies, especially multicenter RCTs are crucial to determine the added value of RPS.
Acknowledgments
We are appreciated for the help of the audience group members: Xuan Zhang; Guodong Zhao; Xiaodong Tan; Tao Jiang; Bin Han; Yuanxin Gao; Zhuzeng Yin; Ning Zhang; Yong Xu; Dabin Xu; Yang Wang; Wenbo Tang; Kedi Zhang; Nan Jiang; Chao Lin.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CREDES reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-132/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-132/coif). M.A.H., F.P. and H.S.H. serve as unpaid editorial board members of Hepatobiliary Surgery and Nutrition. M.G.B. acknowledges grants support to his hospital for the investigator-initiated DIPLOMA-2 randomized trial. B.K.P.G. reports grant from Intuitive Foundation and consulting fees from Transmedic, Local distributor of the Da Vinci robot. U.B. is proctor for Intuitive Surgical (pancreas surgery). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
*Members of Consensus Steering Group: Rong Liu, Mohammed Abu Hila, Marc G. Besselink, Thilo Hackert, Chinnusamy Palanivelu, Yupei Zhao. Members of Consensus Development Group: Jin He, Ugo Boggi, Jin-Young Jang, Panaro Fabrizio, Brian K. P. Goh, Mikhail Efanov, Yuichi Nagakawa, Hong-Jin Kim, Xiaoyu Yin, Zhiming Zhao, Yi-Ming Shyr, Shridhar Iyer, Eli Kakiashvili, Ho-Seong Han, Jae Hoon Lee, Roland Croner, Shin-E Wang, Marco Vito Marino, Arun Prasad, Wei Wang, Songqing He, Tess M. E. van Ramshorst, Kehu Yang. Members of Consensus Secretary Group: Qu Liu, Zizheng Wang, Mengyang Li, Shuai Xu, Kongyuan Wei, Zhaoda Deng, Yuze Jia.
References
- Liu R, Zhao GD, Tang WB, et al. A single-team experience with robotic pancreatic surgery in 1010 cases. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:130-4. [Crossref] [PubMed]
- Guerra F, Checcacci P, Vegni A, et al. Surgical and oncological outcomes of our first 59 cases of robotic pancreaticoduodenectomy. J Visc Surg 2019;156:185-90. [Crossref] [PubMed]
- Piedimonte S, Wang Y, Bergman S, et al. Early experience with robotic pancreatic surgery in a Canadian institution. Can J Surg 2015;58:394-401. [Crossref] [PubMed]
- Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 2016;401:1111-22. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. [Crossref] [PubMed]
- Lee SH, Kang CM, Hwang HK, et al. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc 2014;28:2848-55. [Crossref] [PubMed]
- Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 2011;40:1264-70. [Crossref] [PubMed]
- Liu R, Wakabayashi G, Palanivelu C, et al. International consensus statement on robotic pancreatic surgery. Hepatobiliary Surg Nutr 2019;8:345-60. [Crossref] [PubMed]
- Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg 2020;271:1-14. [Crossref] [PubMed]
- Salibi PN, Martinie JB. Robotic pancreatic surgery: a slowly developing field. Hepatobiliary Surg Nutr 2020;9:673-5. [Crossref] [PubMed]
- Gumbs AA, Croner R, Chouillard E. Is robotic pancreatic surgery finally ready for prime-time? Hepatobiliary Surg Nutr 2020;9:650-3. [Crossref] [PubMed]
- Weng Y, Jin J, Huo Z, et al. Robotic-assisted versus open distal pancreatectomy for benign and low-grade malignant pancreatic tumors: a propensity score-matched study. Surg Endosc 2021;35:2255-64. [Crossref] [PubMed]
- Morelli L, Di Franco G, Guadagni S, et al. Robotic-assisted versus open left pancreatectomy for cystic tumours: A single-centre experience. J Minim Access Surg 2020;16:66-70. [Crossref] [PubMed]
- de Rooij T, van Hilst J, van Santvoort H, et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg 2019;269:2-9. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Klompmaker S, et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): A Pan-European Propensity Score Matched Study. Ann Surg 2019;269:10-7. [Crossref] [PubMed]
- van Hilst J, Korrel M, Lof S, et al. Minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma (DIPLOMA): study protocol for a randomized controlled trial. Trials 2021;22:608. [Crossref] [PubMed]
- Feng Q, Jiang C, Feng X, et al. Robotic Versus Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:752236. [Crossref] [PubMed]
- Hong S, Song KB, Madkhali AA, et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: a single surgeon's experience of 228 consecutive cases. Surg Endosc 2020;34:2465-73. [Crossref] [PubMed]
- Watson MD, Baimas-George MR, Thompson KJ, et al. Improved oncologic outcomes for minimally invasive left pancreatectomy: Propensity-score matched analysis of the National Cancer Database. J Surg Oncol 2020;122:1383-92. [Crossref] [PubMed]
- Korrel M, Roelofs A, van Hilst J, et al. Long-Term Quality of Life after Minimally Invasive vs Open Distal Pancreatectomy in the LEOPARD Randomized Trial. J Am Coll Surg 2021;233:730-739.e9. [Crossref] [PubMed]
- Di Martino M, Caruso R, D'Ovidio A, et al. Robotic versus laparoscopic distal pancreatectomies: A systematic review and meta-analysis on costs and perioperative outcome. Int J Med Robot 2021;17:e2295. [Crossref] [PubMed]
- Xu SB, Jia CK, Wang JR, et al. Do patients benefit more from robot assisted approach than conventional laparoscopic distal pancreatectomy? A meta-analysis of perioperative and economic outcomes. J Formos Med Assoc 2019;118:268-78. [Crossref] [PubMed]
- Lof S, van der Heijde N, Abuawwad M, et al. Robotic versus laparoscopic distal pancreatectomy: multicentre analysis. Br J Surg 2021;108:188-95. [Crossref] [PubMed]
- Zhou J, Lv Z, Zou H, et al. Up-to-date comparison of robotic-assisted versus open distal pancreatectomy: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2020;99:e20435. [Crossref] [PubMed]
- Lyu Y, Cheng Y, Wang B, et al. Comparison of 3 Minimally Invasive Methods Versus Open Distal Pancreatectomy: A Systematic Review and Network Meta-Analysis. Surg Laparosc Endosc Percutan Tech 2020;31:104-12. [Crossref] [PubMed]
- Chen H, Shen Z, Ying X, et al. Robotic distal pancreatectomy reduces pancreatic fistula in patients without visceral obesity as compared to open distal pancreatectomy: A propensity score matching retrospective cohort study. Int J Surg 2021;90:105960. [Crossref] [PubMed]
- Shakir M, Boone BA, Polanco PM, et al. The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. HPB (Oxford) 2015;17:580-6. [Crossref] [PubMed]
- Shyr BU, Chen SC, Shyr YM, et al. Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine (Baltimore) 2018;97:e13000. [Crossref] [PubMed]
- Al Abbas AI, Wang C, Hamad AB, et al. Mentorship and formal robotic proficiency skills curriculum improve subsequent generations' learning curve for the robotic distal pancreatectomy. HPB (Oxford) 2021;23:1849-55. [Crossref] [PubMed]
- Rompianesi G, Montalti R, Ambrosio L, et al. Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: Systematic Review and Meta-Analysis. J Pers Med 2021;11:552. [Crossref] [PubMed]
- Esposito A, Casetti L, De Pastena M, et al. Robotic spleen-preserving distal pancreatectomy: the Verona experience. Updates Surg 2021;73:923-8. [Crossref] [PubMed]
- Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18. [Crossref] [PubMed]
- Eckhardt S, Schicker C, Maurer E, et al. Robotic-Assisted Approach Improves Vessel Preservation in Spleen-Preserving Distal Pancreatectomy. Dig Surg 2016;33:406-13. [Crossref] [PubMed]
- Liu R, Liu Q, Zhao ZM, et al. Robotic versus laparoscopic distal pancreatectomy: A propensity score-matched study. J Surg Oncol 2017;116:461-9. [Crossref] [PubMed]
- Kwon J, Lee JH, Park SY, et al. A comparison of robotic versus laparoscopic distal pancreatectomy: Propensity score matching analysis. Int J Med Robot 2022;18:e2347. [Crossref] [PubMed]
- Chee M, Lee CY, Lee SY, et al. Short- and long-term outcomes after minimally invasive versus open spleen-saving distal pancreatectomies. J Minim Access Surg 2022;18:118-24. [Crossref] [PubMed]
- Shi Y, Wang Q, Shi Z, et al. Comparison between robot-assisted middle pancreatectomy and robot-assisted distal pancreatectomy for benign or low-grade malignant tumours located in the neck of the pancreas: A propensity score matched study. Int J Med Robot 2021;17:e2219. [Crossref] [PubMed]
- Wang ZZ, Zhao GD, Zhao ZM, et al. A comparative study of end-to-end pancreatic anastomosis versus pancreaticojejunostomy after robotic central pancreatectomy. Updates Surg 2021;73:967-75. [Crossref] [PubMed]
- Huynh F, Cruz CJ, Hwang HK, et al. Minimally invasive (laparoscopic and robot-assisted) versus open approach for central pancreatectomies: a single-center experience. Surg Endosc 2022;36:1326-31. [Crossref] [PubMed]
- Shi Y, Jin J, Huo Z, et al. An 8-year single-center study: 170 cases of middle pancreatectomy, including 110 cases of robot-assisted middle pancreatectomy. Surgery 2020;167:436-41. [Crossref] [PubMed]
- Rompianesi G, Montalti R, Giglio MC, et al. Robotic central pancreatectomy: a systematic review and meta-analysis. HPB (Oxford) 2022;24:143-51. [Crossref] [PubMed]
- Jung D, Bari H, Hwang HK, et al. Short and long-term outcomes of minimally invasive central pancreatectomy: Comparison with minimally invasive spleen-preserving subtotal distal pancreatectomy. Asian J Surg 2023;46:824-8. [Crossref] [PubMed]
- Shi Y, Wang Y, Wang J, et al. Learning curve of robot-assisted middle pancreatectomy (RMP): experience of the first 100 cases from a high-volume pancreatic center in China. Surg Endosc 2020;34:3513-20. [Crossref] [PubMed]
- Kabir T, Tan HL, Syn NL, et al. Outcomes of laparoscopic, robotic, and open pancreatoduodenectomy: A network meta-analysis of randomized controlled trials and propensity-score matched studies. Surgery 2022;171:476-89. [Crossref] [PubMed]
- Zhang W, Huang Z, Zhang J, et al. Safety and efficacy of robot-assisted versus open pancreaticoduodenectomy: a meta-analysis of multiple worldwide centers. Updates Surg 2021;73:893-907. [Crossref] [PubMed]
- Xu DB, Zhao ZM, Xu Y, et al. Hybrid pancreatoduodenectomy in laparoscopic and robotic surgery: a single-center experience in China. Surg Endosc 2021;35:1703-12. [Crossref] [PubMed]
- Vining CC, Kuchta K, Berger Y, et al. Robotic pancreaticoduodenectomy decreases the risk of clinically relevant post-operative pancreatic fistula: a propensity score matched NSQIP analysis. HPB (Oxford) 2021;23:367-78. [Crossref] [PubMed]
- van Oosten AF, Ding D, Habib JR, et al. Perioperative Outcomes of Robotic Pancreaticoduodenectomy: a Propensity-Matched Analysis to Open and Laparoscopic Pancreaticoduodenectomy. J Gastrointest Surg 2021;25:1795-804. [Crossref] [PubMed]
- Shyr BU, Shyr BS, Chen SC, et al. Robotic and open pancreaticoduodenectomy: results from Taipei Veterans General Hospital in Taiwan. Updates Surg 2021;73:939-46. [Crossref] [PubMed]
- Mulchandani J, Shetty N, Kulkarni A, et al. Short-term and pathologic outcomes of robotic versus open pancreatoduodenectomy for periampullary and pancreatic head malignancy: an early experience. J Robot Surg 2022;16:859-66. [Crossref] [PubMed]
- Podda M, Gerardi C, Di Saverio S, et al. Robotic-assisted versus open pancreaticoduodenectomy for patients with benign and malignant periampullary disease: a systematic review and meta-analysis of short-term outcomes. Surg Endosc 2020;34:2390-409. [Crossref] [PubMed]
- Bencini L, Tofani F, Paolini C, et al. Single-centre comparison of robotic and open pancreatoduodenectomy: a propensity score-matched study. Surg Endosc 2020;34:5402-12. [Crossref] [PubMed]
- Shyr BU, Shyr BS, Chen SC, et al. Propensity score-matched comparison of the oncological feasibility and survival outcomes for pancreatic adenocarcinoma with robotic and open pancreatoduodenectomy. Surg Endosc 2022;36:1507-14. [Crossref] [PubMed]
- Magistri P, Boggi U, Esposito A, et al. Robotic vs open distal pancreatectomy: A multi-institutional matched comparison analysis. J Hepatobiliary Pancreat Sci 2021;28:1098-106. [Crossref] [PubMed]
- Weng Y, Jiang Y, Fu N, et al. Oncological outcomes of robotic-assisted versus open pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a propensity score-matched analysis. Surg Endosc 2021;35:3437-48. [Crossref] [PubMed]
- Sun R, Yu J, Zhang Y, et al. Perioperative and oncological outcomes following minimally invasive versus open pancreaticoduodenectomy for pancreatic duct adenocarcinoma. Surg Endosc 2021;35:2273-85. [Crossref] [PubMed]
- Wang W, Liu Q, Zhao ZM, et al. Comparison of robotic and open pancreaticoduodenectomy for primary nonampullary duodenal adenocarcinoma: a retrospective cohort study. Langenbecks Arch Surg 2022;407:167-73. [Crossref] [PubMed]
- Zhang XP, Xu S, Wang Y, et al. Robotic pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: A large-scale study. J Hepatobiliary Pancreat Sci 2021;28:942-52. [Crossref] [PubMed]
- Liu Q, Zhao Z, Zhang X, et al. Perioperative and Oncological Outcomes of Robotic Versus Open Pancreaticoduodenectomy in Low-Risk Surgical Candidates: A Multicenter Propensity Score-Matched Study. Ann Surg 2023;277:e864-71. [Crossref] [PubMed]
- Da Dong X, Felsenreich DM, Gogna S, et al. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep 2021;11:3774. [Crossref] [PubMed]
- Jin J, Yin SM, Weng Y, et al. Robotic versus open pancreaticoduodenectomy with vascular resection for pancreatic ductal adenocarcinoma: surgical and oncological outcomes from pilot experience. Langenbecks Arch Surg 2022;407:1489-97. [Crossref] [PubMed]
- Shyr BU, Chen SC, Shyr YM, et al. Surgical, survival, and oncological outcomes after vascular resection in robotic and open pancreaticoduodenectomy. Surg Endosc 2020;34:377-83. [Crossref] [PubMed]
- Nassour I, Tohme S, Hoehn R, et al. Safety and oncologic efficacy of robotic compared to open pancreaticoduodenectomy after neoadjuvant chemotherapy for pancreatic cancer. Surg Endosc 2021;35:2248-54. [Crossref] [PubMed]
- Park SE, Choi HJ, You YK, et al. Effectiveness and stability of robot-assisted anastomosis in minimally invasive pancreaticoduodenectomy. Ann Surg Treat Res 2021;100:329-37. [Crossref] [PubMed]
- Kim H, Choi SH, Jang JY, et al. Multicenter comparison of totally laparoscopic and totally robotic pancreaticoduodenectomy: Propensity score and learning curve-matching analyses. J Hepatobiliary Pancreat Sci 2022;29:311-21. [Crossref] [PubMed]
- Napoli N, Kauffmann EF, Menonna F, et al. Robotic versus open pancreatoduodenectomy: a propensity score-matched analysis based on factors predictive of postoperative pancreatic fistula. Surg Endosc 2018;32:1234-47. [Crossref] [PubMed]
- Chao YJ, Liao TK, Su PJ, et al. Impact of body mass index on the early experience of robotic pancreaticoduodenectomy. Updates Surg 2021;73:929-37. [Crossref] [PubMed]
- Liu Q, Zhao Z, Zhang X, et al. Robotic pancreaticoduodenectomy in elderly and younger patients: A retrospective cohort study. Int J Surg 2020;81:61-5. [Crossref] [PubMed]
- Zureikat AH, Beane JD, Zenati MS, et al. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Ann Surg 2021;273:966-72. [Crossref] [PubMed]
- Zwart MJW, Nota CLM, de Rooij T, et al. Outcomes of a Multicenter Training Program in Robotic Pancreatoduodenectomy (LAELAPS-3). Ann Surg 2022;276:e886-95. [Crossref] [PubMed]
- Schmidt CR, Harris BR, Musgrove KA, et al. Formal robotic training diminishes the learning curve for robotic pancreatoduodenectomy: Implications for new programs in complex robotic surgery. J Surg Oncol 2021;123:375-80. [Crossref] [PubMed]
- Ryoo DY, Eskander MF, Hamad A, et al. Mitigation of the Robotic Pancreaticoduodenectomy Learning Curve through comprehensive training. HPB (Oxford) 2021;23:1550-6. [Crossref] [PubMed]
- Rosemurgy A, Ross S, Bourdeau T, et al. Cost Analysis of Pancreaticoduodenectomy at a High-Volume Robotic Hepatopancreaticobiliary Surgery Program. J Am Coll Surg 2021;232:461-9. [Crossref] [PubMed]
- Di Franco G, Lorenzoni V, Palmeri M, et al. Robot-assisted pancreatoduodenectomy with the da Vinci Xi: can the costs of advanced technology be offset by clinical advantages? A case-matched cost analysis versus open approach. Surg Endosc 2022;36:4417-28. [Crossref] [PubMed]
- Jin J, Shi Y, Chen M, et al. Robotic versus Open Pancreatoduodenectomy for Pancreatic and Periampullary Tumors (PORTAL): a study protocol for a multicenter phase III non-inferiority randomized controlled trial. Trials 2021;22:954. [Crossref] [PubMed]
- Klotz R, Dörr-Harim C, Bruckner T, et al. Evaluation of robotic versus open partial pancreatoduodenectomy-study protocol for a randomised controlled pilot trial (EUROPA, DRKS00020407). Trials 2021;22:40. [Crossref] [PubMed]
- Yan Q, Xu LB, Ren ZF, et al. Robotic versus open pancreaticoduodenectomy: a meta-analysis of short-term outcomes. Surg Endosc 2020;34:501-9. [Crossref] [PubMed]
- Baimas-George M, Watson M, Murphy KJ, et al. Robotic pancreaticoduodenectomy may offer improved oncologic outcomes over open surgery: a propensity-matched single-institution study. Surg Endosc 2020;34:3644-9. [Crossref] [PubMed]
- Kamarajah SK, Bundred JR, Marc OS, et al. A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB (Oxford) 2020;22:329-39. [Crossref] [PubMed]
- Zhao W, Liu C, Li S, et al. Safety and efficacy for robot-assisted versus open pancreaticoduodenectomy and distal pancreatectomy: A systematic review and meta-analysis. Surg Oncol 2018;27:468-78. [Crossref] [PubMed]
- Aiolfi A, Lombardo F, Bonitta G, et al. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg 2021;73:909-22. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Lee PY, Shyr BU, Shyr BS, et al. Surgical and survival outcomes after robotic and open pancreaticoduodenectomy with positive margins. J Chin Med Assoc 2021;84:698-703. [Crossref] [PubMed]
- Peng L, Lin S, Li Y, et al. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surg Endosc 2017;31:3085-97. [Crossref] [PubMed]
- Olakowski M, Grudzińska E. Pancreatic head cancer - Current surgery techniques. Asian J Surg 2023;46:73-81. [Crossref] [PubMed]
- Kauffmann EF, Napoli N, Menonna F, et al. A propensity score-matched analysis of robotic versus open pancreatoduodenectomy for pancreatic cancer based on margin status. Surg Endosc 2019;33:234-42. [Crossref] [PubMed]
- Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. [Crossref] [PubMed]
- Chan KS, Wang ZK, Syn N, et al. Learning curve of laparoscopic and robotic pancreas resections: a systematic review. Surgery 2021;170:194-206. [Crossref] [PubMed]
- Tyutyunnik P, Klompmaker S, Lombardo C, et al. Learning curve of three European centers in laparoscopic, hybrid laparoscopic, and robotic pancreatoduodenectomy. Surg Endosc 2022;36:1515-26. [Crossref] [PubMed]
- Goh BKP, Low TY, Lee SY, et al. Initial experience with robotic pancreatic surgery in Singapore: single institution experience with 30 consecutive cases. ANZ J Surg 2019;89:206-10. [Crossref] [PubMed]
- Beane JD, Zenati M, Hamad A, et al. Robotic pancreatoduodenectomy with vascular resection: Outcomes and learning curve. Surgery 2019;166:8-14. [Crossref] [PubMed]
- Han DH, Kang CM, Lee WJ, et al. A five-year survivor without recurrence following robotic anterior radical antegrade modular pancreatosplenectomy for a well-selected left-sided pancreatic cancer. Yonsei Med J 2014;55:276-9. [Crossref] [PubMed]
- Napoli N, Kauffmann EF, Menonna F, et al. Robot-Assisted Radical Antegrade Modular Pancreatosplenectomy Including Resection and Reconstruction of the Spleno-Mesenteric Junction. J Vis Exp 2020; [Crossref]
- Takagi K, Umeda Y, Yoshida R, et al. Robotic Radical Antegrade Modular Pancreatosplenectomy Using the Supracolic Anterior Superior Mesenteric Artery Approach. J Gastrointest Surg 2021;25:3015-8. [Crossref] [PubMed]
- Liu Q, Zhao G, Zhao Z, et al. The standardized technique in robotic radical antegrade modular pancreatosplenectomy using the flip-up approach. Langenbecks Arch Surg 2021;406:1697-703. [Crossref] [PubMed]
- Li M, Liu Q, Zhang T, et al. Evaluating the learning curve of robotic radical antegrade modular pancreatosplenectomy: A retrospective cohort study. Int J Surg 2022;101:106612. [Crossref] [PubMed]
- Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. [Crossref] [PubMed]
- Kaçmaz E, Zwart MJW, Engelsman AF, et al. Robotic Enucleation of an Intra-Pancreatic Insulinoma in the Pancreatic Head. J Vis Exp 2020; [Crossref]
- Schulte Am Esch J, Krüger M, Barthlen W, et al. Technical aspects of paediatric robotic pancreatic enucleation based on a case of an insulinoma. Int J Med Robot 2021;17:e2317. [Crossref] [PubMed]
- Najafi N, Mintziras I, Wiese D, et al. A retrospective comparison of robotic versus laparoscopic distal resection and enucleation for potentially benign pancreatic neoplasms. Surg Today 2020;50:872-80. [Crossref] [PubMed]
- Shi Y, Peng C, Shen B, et al. Pancreatic enucleation using the da Vinci robotic surgical system: a report of 26 cases. Int J Med Robot 2016;12:751-7. [Crossref] [PubMed]
- Jin JB, Qin K, Li H, et al. Robotic Enucleation for Benign or Borderline Tumours of the Pancreas: A Retrospective Analysis and Comparison from a High-Volume Centre in Asia. World J Surg 2016;40:3009-20. [Crossref] [PubMed]
- Guerra F, Giuliani G, Bencini L, et al. Minimally invasive versus open pancreatic enucleation. Systematic review and meta-analysis of surgical outcomes. J Surg Oncol 2018;117:1509-16. [Crossref] [PubMed]
- Dalla Valle R, Cremaschi E, Lamecchi L, et al. Open and minimally invasive pancreatic neoplasms enucleation: a systematic review. Surg Endosc 2019;33:3192-9. [Crossref] [PubMed]
- Souche R, Herrero A, Bourel G, et al. Robotic versus laparoscopic distal pancreatectomy: a French prospective single-center experience and cost-effectiveness analysis. Surg Endosc 2018;32:3562-9. [Crossref] [PubMed]
- Rodriguez M, Memeo R, Leon P, et al. Which method of distal pancreatectomy is cost-effective among open, laparoscopic, or robotic surgery? Hepatobiliary Surg Nutr 2018;7:345-52. [Crossref] [PubMed]
- Vicente E, Núñez-Alfonsel J, Ielpo B, et al. A cost-effectiveness analysis of robotic versus laparoscopic distal pancreatectomy. Int J Med Robot 2020;16:e2080. [Crossref] [PubMed]
- Partelli S, Ricci C, Cinelli L, et al. Evaluation of cost-effectiveness among open, laparoscopic and robotic distal pancreatectomy: A systematic review and meta-analysis. Am J Surg 2021;222:513-20. [Crossref] [PubMed]
- Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148:814-23. [Crossref] [PubMed]
- Magge DR, Zenati MS, Hamad A, et al. Comprehensive comparative analysis of cost-effectiveness and perioperative outcomes between open, laparoscopic, and robotic distal pancreatectomy. HPB (Oxford) 2018;20:1172-80. [Crossref] [PubMed]
- Fisher AV, Fernandes-Taylor S, Schumacher JR, et al. Analysis of 90-day cost for open versus minimally invasive distal pancreatectomy. HPB (Oxford) 2019;21:60-6. [Crossref] [PubMed]
- Benzing C, Timmermann L, Winklmann T, et al. Robotic versus open pancreatic surgery: a propensity score-matched cost-effectiveness analysis. Langenbecks Arch Surg 2022;407:1923-33. [Crossref] [PubMed]
- De Pastena M, Esposito A, Paiella S, et al. Cost-effectiveness and quality of life analysis of laparoscopic and robotic distal pancreatectomy: a propensity score-matched study. Surg Endosc 2021;35:1420-8. [Crossref] [PubMed]
- Donahue TR, Reber HA. Pancreatic surgery. Curr Opin Gastroenterol 2013;29:552-8. [Crossref] [PubMed]
- Fujino Y. Perioperative management of distal pancreatectomy. World J Gastroenterol 2015;21:3166-9. [Crossref] [PubMed]
- Jiang L, Ning D, Chen XP. Improvement in distal pancreatectomy for tumors in the body and tail of the pancreas. World J Surg Oncol 2021;19:49. [Crossref] [PubMed]
- Moraldi L, Pesi B, Bencini L, et al. Robotic distal pancreatectomy with selective closure of pancreatic duct: surgical outcomes. Updates Surg 2019;71:145-50. [Crossref] [PubMed]
- Goh BKP, Lee CY, Koh YX, et al. Use of Reinforced Staplers Decreases the Rate of Postoperative Pancreatic Fistula Compared to Bare Staplers After Minimally Invasive Distal Pancreatectomies. J Laparoendosc Adv Surg Tech A 2021;31:1124-9. [Crossref] [PubMed]
- Heeger K, Falconi M, Partelli S, et al. Increased rate of clinically relevant pancreatic fistula after deep enucleation of small pancreatic tumors. Langenbecks Arch Surg 2014;399:315-21. [Crossref] [PubMed]
- Giuliani T, Marchegiani G, Girgis MD, et al. Endoscopic placement of pancreatic stent for "Deep" pancreatic enucleations operative technique and preliminary experience at two high-volume centers. Surg Endosc 2020;34:2796-802. [Crossref] [PubMed]
- Malgras B, Douard R, Siauve N, et al. Management of left pancreatic trauma. Am Surg 2011;77:1-9.
- Ando Y, Okano K, Yasumatsu H, et al. Current status and management of pancreatic trauma with main pancreatic duct injury: A multicenter nationwide survey in Japan. J Hepatobiliary Pancreat Sci 2021;28:183-91. [Crossref] [PubMed]
- Zwart MJW, Jones LR, Balduzzi A, et al. Added value of 3D-vision during robotic pancreatoduodenectomy anastomoses in biotissue (LAEBOT 3D2D): a randomized controlled cross-over trial. Surg Endosc 2021;35:2928-35. [Crossref] [PubMed]
- Wang SE, Shyr BU, Chen SC, et al. Comparison between robotic and open pancreaticoduodenectomy with modified Blumgart pancreaticojejunostomy: A propensity score-matched study. Surgery 2018;164:1162-7. [Crossref] [PubMed]
- Menonna F, Napoli N, Kauffmann EF, et al. Additional modifications to the Blumgart pancreaticojejunostomy: Results of a propensity score-matched analysis versus Cattel-Warren pancreaticojejunostomy. Surgery 2021;169:954-62. [Crossref] [PubMed]
- Giulianotti PC, Mangano A, Bustos RE, et al. Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique: Lessons learned since the first worldwide RPD performed in the year 2001. Surg Endosc 2018;32:4329-36. [Crossref] [PubMed]
- Giulianotti PC, Gonzalez-Heredia R, Esposito S, et al. Trans-gastric pancreaticogastrostomy reconstruction after pylorus-preserving robotic Whipple: a proposal for a standardized technique. Surg Endosc 2018;32:2169-74. [Crossref] [PubMed]
- Cheng Y, Briarava M, Lai M, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev 2017;9:CD012257. [Crossref] [PubMed]
- Liu Q, Zhao Z, Gao Y, et al. Novel single-layer continuous suture of pancreaticojejunostomy for robotic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2020;27:56-63. [Crossref] [PubMed]
- Liu Q, Zhao Z, Gao Y, et al. Novel Technique for Single-Layer Pancreatojejunostomy is Not Inferior to Modified Blumgart Anastomosis in Robotic Pancreatoduodenectomy: Results of a Randomized Controlled Trial. Ann Surg Oncol 2021;28:2346-55. [Crossref] [PubMed]
- Kiguchi G, Sugioka A, Uchida Y, et al. Wrapping double-mattress anastomosis for pancreaticojejunostomy in minimally invasive pancreaticoduodenectomy can significantly reduce postoperative pancreatic fistula rate compared with conventional pancreaticojejunostomy in open surgery: An analysis of a propensity score-matched sample. Surg Oncol 2021;38:101577. [Crossref] [PubMed]
- Marino MV, Heng Chiow AK, Mirabella A, et al. Rate of Post-Operative Pancreatic Fistula after Robotic-Assisted Pancreaticoduodenectomy with Pancreato-Jejunostomy versus Pancreato-Gastrostomy: A Retrospective Case Matched Comparative Study. J Clin Med 2021;10:2181. [Crossref] [PubMed]
- Nassour I, Wang SC, Porembka MR, et al. Conversion of Minimally Invasive Distal Pancreatectomy: Predictors and Outcomes. Ann Surg Oncol 2017;24:3725-31. [Crossref] [PubMed]
- Nassour I, Wang SC, Porembka MR, et al. Robotic Versus Laparoscopic Pancreaticoduodenectomy: a NSQIP Analysis. J Gastrointest Surg 2017;21:1784-92. [Crossref] [PubMed]
- Kamarajah SK, Bundred J, Marc OS, et al. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol 2020;46:6-14. [Crossref] [PubMed]
- de Rooij T, van Hilst J, Boerma D, et al. Impact of a Nationwide Training Program in Minimally Invasive Distal Pancreatectomy (LAELAPS). Ann Surg 2016;264:754-62. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Kamarajah SK, Sutandi N, Robinson SR, et al. Robotic versus conventional laparoscopic distal pancreatic resection: a systematic review and meta-analysis. HPB (Oxford) 2019;21:1107-18. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Halls MC, Cipriani F, Berardi G, et al. Conversion for Unfavorable Intraoperative Events Results in Significantly Worse Outcomes During Laparoscopic Liver Resection: Lessons Learned From a Multicenter Review of 2861 Cases. Ann Surg 2018;268:1051-7. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford) 2016;18:835-42. [Crossref] [PubMed]
- Krell RW, McNeil LR, Yanala UR, et al. Neoadjuvant Therapy for Pancreatic Ductal Adenocarcinoma: Propensity-Matched Analysis of Postoperative Complications Using ACS-NSQIP. Ann Surg Oncol 2021;28:3810-22. [Crossref] [PubMed]
- AlMasri S, Paniccia A, Zureikat AH. Robotic Pancreaticoduodenectomy for a Technically Challenging Pancreatic Head Cancer. J Gastrointest Surg 2021;25:1359. [Crossref] [PubMed]
- Hank T, Sandini M, Ferrone CR, et al. Association Between Pancreatic Fistula and Long-term Survival in the Era of Neoadjuvant Chemotherapy. JAMA Surg 2019;154:943-51. [Crossref] [PubMed]