Physical activity decreases in patients on the liver transplant waiting list and influences postoperative outcome—a prospective cohort study
Highlight box
Key findings
• Over time, there is a significant decrease of moderate physical activity (MPA) after week 40 on wait list for liver transplantation (LT). No association of MPA over time with age, Child-Pugh Score, Liver Frailty Index or quality of life at time of inclusion to study.
• Female patients and patients receiving nutritional support had an increase in MPA (week 20 and 40).
• Reduced MPA is significantly associated with adverse outcome post-LT.
What is known and what is new?
• Evidence in other surgical fields suggests that pre-habilitation is beneficial in reducing post-surgical morbidity and mortality.
• First prospective study evaluating specifically pretransplant physical activity using wearable device demonstrating an association with adverse outcomes in this cohort with a significant sex-related disparity.
What is the implication, and what should change now?
• This study provides evidence for warranted interventional studies on physical activity combined with nutritional support in pre-LT.
Introduction
Background
Decreased physical performance or deconditioning [as measured by e.g., skeletal muscle index (1-3), handgrip strength (1), 6-minute walk distance (4)], associated sarcopenia (2,5) and frailty occur in up to 78% of patients (3) with end-stage liver disease (ESLD). They are considered multifactorial and correlate with morbidity and mortality (6-10). Specifically, sarcopenia and frailty have been shown to be associated with increased mortality in patients with ESLD listed for liver transplantation (LT) during the waiting list period (8,11) as well as post-LT (12). A sedentary behavior [<1.5 metabolic equivalents of tasks (METs) daily] is observed in 75.9%±18.9% of patients awaiting LT (8). Moreover, persistent deconditioning post-LT (13) is associated with decreased quality of life (QoL) (14), morbidity and mortality (15). A recent meta-analysis assessing eight randomized control trials, evaluating physical rehabilitation after LT, was able to show improved cardiorespiratory fitness and QoL (16).
Rationale and knowledge gap
In the general population, growing evidence shows that pre-habilitation can be beneficial, leading to improved stress tolerance (17), and better surgical outcomes (18,19), impacting on tissue regeneration (20) and metabolism (21) as well as mental health (22). However, controversies regarding studied populations exist, resulting in a debate concerning the significance of pre-habilitation (19,23,24). Independent of surgery, the World Health Organization (WHO) recommends 150–300 min of moderate physical activity (MPA) weekly (25). Whilst pre-habilitation before LT is strongly recommended in the 2022 International Liver Transplantation Society consensus guidelines, the quality of evidence remains very low (26).
Objective
We hypothesized that (I) the majority (≥50%) of patients pre-LT do not meet the WHO recommendations of MPA (25), (II) that Liver Frailty Index (LFI) (27) at LT listing is significantly associated with a change of PA over time, and (III) decreased pretransplant MPA is associated with postoperative adverse clinical outcome. With this prospective cohort study, we (I) describe the PA with regard to WHO recommendations in patients wait-listed for LT in a real-life setting, (II) assess factors influencing the PA and its change over time and (III) test the association of pretransplant MPA with postoperative morbidity and mortality. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-399/rc).
Methods
Study design
We conducted a single center prospective observational longitudinal study at the Department of Visceral Surgery and Medicine, Inselspital, University Hospital Bern in Switzerland. All patients included in the present study signed a specific informed consent. The study was approved by the local ethics committee (Kantonale Ethikkommission, Kanton Bern, BASEC ID 2019-00606). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Selection of patients
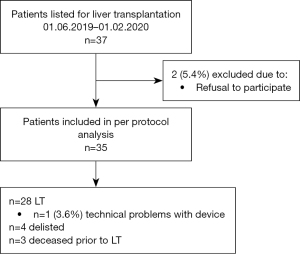
The consecutive recruitment was initiated in June 2019 and was prematurely halted in February 2020 due to the SARS-CoV-2 pandemic. Initial planned sample-size was 40 patients. Follow-up ended at six months after LT, whereas patients who had not yet been transplanted, were followed up regularly every six months. Eligible patients included adults (≥18 years) who had been accepted on the waiting list for a liver only or combined liver/kidney transplant. Exclusion criteria were failure to provide oral and written informed consent. Participants could discontinue their enrollment at any time point. Reporting of this observational study was undertaken in accordance with the STROBE guidelines and the flowchart is shown in Figure 1.
Activity tracker
For the PA assessment, the patients needed to have access to a cell phone with Bluetooth connection and had to be able to navigate the Fitbit-App. Patients were instructed to continuously wear the activity tracker (Fitbit Charge 2, Fitbit LLC, San Francisco, CA, USA, RRID: non-existant) for the study period. The activity data was downloaded three monthly during the outpatient visits. The automatic stratification of the PA into low, moderate and vigorous occurred according to the wearable trackers’ producers coding. The wearable device was funded by the study center from internal funds. A cumulative weekly (seven days) PA was calculated in order to be in line with WHO recommendations and adjust for daily fluctuations.
Clinical definitions
Frailty was assessed every three months using the LFI (27) and QoL using the SF36 questionnaire every six months (28). Nutritional support was defined as receiving regular counseling by a board-certified dietician in an outpatient setting either at our own hospital or in an institution closer to the patient’s home and oral supplementation either with branch-chained amino acids or other protein-rich supplement prescriptions and/or placement of a nasojejunal feeding tube. Placement of a nasojejunal feeding tube was usually done as an inpatient procedure with enteral feeding started in a hospital setting and once established a supported by the patient, continued as an outpatient with support from community nurses. Frequency of outpatient consultations was carried out based on the individual needs of each patient.
Outcome
The primary endpoint was to describe the weekly moderate PA in patients wait-listed for LT in accordance with the WHO recommendations (25). Secondary outcome measures (factors influencing PA and its change over time and association with composite adverse clinical outcome) were assessed in a longitudinal temporo-spatial analysis. The composite adverse clinical outcome endpoint was defined as the need for re-transplantation, emergency hospitalization or occurrence of in-hospital complications during LT as defined per the Dindo-Clavien classification ≥ IIIa (29,30) occurring within the first six months post-LT.
Statistical analysis
Categorical variables were reported as numbers and percentages, continuous variables as medians and interquartile ranges (IQRs). Categorical variables were compared using Fisher’s exact test. Continuous variables were assessed using Mann-Whitney U-test. A P of 0.05 or less was considered statistically significant. Temporo-spatial data visualization was done using non-parametric LOESS regression and heatmap displaying medians in boxes of 5 weeks and MPA 20 min/week.
Complex statistical analyses were carried out with the help of a biostatistician. Due to the skewed distribution of the weekly moderate PA data, mixed-effects generalized linear models with a gamma distribution and a log link were used. Splines and time periods were used to have more flexibility modelling the data with two knots, one at week 20 and a second at week 40. The estimation of the association was done for these three time periods (0 to 20, 20 to 40 and after 40 weeks onward). Random slope and intercept were included in the model. Robust estimation of the variance was used. Weekly MPA values of zero were excluded from further analysis. First, a mixed-effects generalized linear model with a Gamma distribution and a log link was used to analyze weekly MPA (time included through three splines as dependent variable). Second, the same model was used to analyze the univariate impact of factors (a priori per-protocol analysis with variables at time of inclusion: categorical: sex, nutritional support and continuous variables: age, Child-Pugh Score, LFI, SF36 total score) on the spatial weekly moderate PA. Finally, a logistic regression was used to estimate the odds ratio of the occurrence of the composite clinical outcomes in dependence of the weekly moderate PA. Cluster estimation of the variance was performed for each patient.
Statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria, RRID: SCR_001905) with R Studio software (R Studio, Inc, Boston, MA, USA; RRID: SCR_000432) and Stata 17 (Stata Corporation, College Station, TX, USA; RRID: SCR_012763).
Results
Patient population
A total of 37 patients were listed for LT during the study period and after evaluation 35 patients were included (Figure 1). Twenty-five (71%) were male with a median age 59 years (IQR, 51.0–63.0 years) (Table 1). Most common primary indication for LT was 14 (40%) hepatocellular carcinoma (HCC). At time of inclusion into the study 21 (60%) of patients’ liver function was classified as Child-Pugh-Score B, median LFI was 3.4 (IQR, 3.0–3.8; 11 robust, 17 pre-frail and 5 frail). The median handgrip was 32.8 kg (IQR, 22.6–38.6 kg). During the study period 28 (80%) patients underwent LT, three died before LT and four were delisted (two considered too sick for LT and two improved significantly not requiring LT) (Figure 1). Ninety-day mortality after LT was 0% and composite adverse clinical outcomes occurred in 15 patients (overall 43%, of transplanted 54%) (Table 2).
Table 1
| Characteristics | Value (n=35) |
|---|---|
| Age (years) | 59.0 (51.0, 63.0) |
| Sex | |
| Male | 25 [71] |
| Weight (kg) | 86.0 (71.7, 94.8) |
| BMI (kg/m2) | 28.0 (25.3, 30.0) |
| Bilirubin (mcmol/L) | 24.0 (14.5, 47.5) |
| Creatinine (mcmol/L) | 78.0 (61.5, 107.5) |
| Albumin (g/L) | 34.0 (26.0, 36.5) |
| CPS | |
| CPS A | 10 [29] |
| CPS B | 21 [60] |
| CPS C | 4 [11] |
| Lab MELD at time point study inclusion | 11.0 (8.0, 19.0) |
| Allocation MELD at time point LT | 32.0 (25.5, 37.5) |
| Use of selective beta blocker | 21 [60] |
| History of decompensation | |
| Ascites | 23 [66] |
| Variceal bleeding | 11 [31] |
| Hepatic encephalopathy | 14 [40] |
| Co-morbidities | |
| T2DM | 12 [34] |
| CKD | 9 [26] |
| Hypertension | 7 [20] |
| Heart disease | 6 [17] |
| Pulmonary disease | 6 [17] |
| Metabolic syndrome | 7 [20] |
| Malnutrition | 8 [23] |
| Others | 13 [37] |
| Nutritional support prior to LT | 10 [29] |
| Primary/major indication for LT | |
| Complications of cirrhosis | 14 [40] |
| HCC | 14 [40] |
| Other | 5 [14] |
| Primary liver tumor other than HCC | 2 [6] |
| Underlying liver pathology | |
| ALD/ASH | 8 [23] |
| NASH | 7 [20] |
| NASH/MASH | 7 [20] |
| HCV | 3 [9] |
| AIH/PBC/PSC | 3 [9] |
| Other | 7 [20] |
| Handgrip total (kg) at inclusion | 32.8 (22.6, 38.6) |
| Standing (s) at inclusion | 8.5 (7.8, 12.1) |
| Balancing total (s) at inclusion | 30 (30, 30) |
| Liver Frailty Index at inclusion | 3.4 (3.0, 3.8) |
| Liver class of frailty at inclusion | |
| Robust | 11 [31] |
| Pre-frail | 17 [49] |
| Frail | 5 [14] |
Values are given in numbers [%] for categorical or medians (interquartile ranges) for continuous variables. BMI, body mass index; CPS, Child-Pugh Score; MELD, Model End-Stage Liver Disease; LT, liver transplantation; T2DM, type 2 diabetes mellitus; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; ALD, alcoholic liver disease; ASH, alcoholic steatohepatitis; NASH, non-alcoholic steatohepatitis; MASH, metabolic dysfunction associated fatty liver disease; HCV, hepatitis C virus; AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Table 2
| Characteristics | Value (n=35) |
|---|---|
| LT | 28 (80.0) |
| Time from listing to study inclusion (days) | 88 (11–264) |
| Time from listing to LT (days) | 370 (239–513) |
| Time from study inclusion to LT (days) | 195 (109–350) |
| Pre-transplant period | |
| All hospitalizations (elective and emergency) | 23 (65.7) |
| Emergency hospitalizations | 18 (51.4) |
| De-listing for LT | 4 (11.4) |
| Mortality prior to LT | 3 (8.6) |
| Transplant period | |
| Length of stay (days) | 8.5 (6.0–14.3) |
| ICU length of stay (days) | 2.0 (1.0–3.0) |
| In-hospital complication | |
| Surgical | 13 (37.1) |
| Non-surgical | 1 (2.9) |
| Severity of complication according to Dindo-Clavien-Classification | |
| IIIa | 2 (5.7) |
| IIIb | 5 (14.3) |
| IVa | 4 (11.4) |
| Description of complication | |
| Bleeding | 4 (11.4) |
| Biliary complication | 3 (8.6) |
| Arterial complication | 2 (5.7) |
| PV thrombosis | 2 (5.7) |
| Graft dysfunction | 2 (5.7) |
| Portocaval-shunt-stenosis | 1 (2.9) |
| Post-transplant period | |
| Emergency re-hospitalization | 8 (22.9) |
| Mortality 90 days after LT | 0 |
| Relisting for re-LT | 4 (11.4) |
| Re-LT | 4 (11.4) |
| Indication for re-LT | |
| PV thrombosis | 1 (2.9) |
| Primary non function | 1 (2.9) |
| Biliary complication | 1 (2.9) |
| Other | 1 (2.9) |
| Composite postoperative adverse outcomes (re-LT or post-LT emergency hospitalization or in-hospital complication during LT) | 15 (42.9) |
Values are given in numbers (%) for categorical or medians (interquartile ranges) for continuous variables accordingly. LT, liver transplant; ICU, intensive care unit; PV, portal vein.
PA
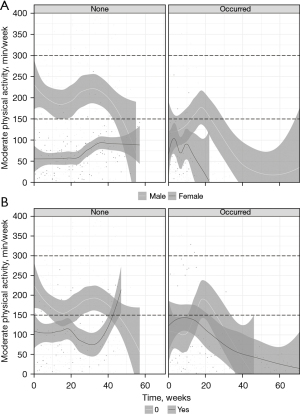
Daily values were tracked and a weekly activity was composed. Daily activity included steps 4,661 (IQR, 1,685–8,609) (Table S1) whereas weekday stratified analysis revealed significant differences of PA measures (P<0.001) with Sunday being the weekday with the least amount of activity (Table S2). A total of 1,053 patient-weeks in 35 patients were recorded (Table 3). The overall median weekly MPA was 41.0 min (IQR, 0.0–127.2 min) with 131 (12%) patient-weeks within the 150–300 min/week category (Table 3). A preliminary mixed linear model analysis found a median decrease of MPA of 0.6 min per week on the waiting list (intercept 111.5 min, correlation −0.800). Longitudinal change is displayed in Figure 2 as a dynamic LOESS regression of all data up until the time of LT. The a priori per-protocol analysis (Table 4) assessing the impact of factors on the longitudinal association with MPA was performed. Temporo-spatial graphical representation of a MPA over time with regard to these a priori factors and stratification per occurrence of the composite adverse clinical endpoint (below) is shown in Figure 3. The visual presentation of the median distribution of continuous factors (age, Child-Pugh Score, LFI and QoL) with regard to MPA and temporal changes are shown using heatmaps in Figure S1. In male patients, a significant decrease of MPA over time was detected only after week 40 after inclusion [regression coefficient (RC) 0.952; 95% confidence interval (CI): 0.926–0.979; P<0.001]. In female patients, a significant increase over time between week 20 and 40 was found (RC 1.025; 95% CI: 1.011–1.039; P<0.001) (Table 4). Sex-associated interaction was identified after week 40 (P<0.001). Patients receiving nutritional support significantly increased MPA between week 20 and 40 (RC 1.011; 95% CI: 1.000–1.022; P=0.045); this decreased again after week 40 (RC 0.962; 95% CI: 0.950–0.974; P<0.001). No significant association was found between age, Child-Pugh Score, LFI or SF36 at time of inclusion and change of MPA over time. A non-significant tendency was observed for SF36 between week 0 to 20 after inclusion (RC 0.998; 95% CI: 0.997–1.000; P=0.095).
Table 3
| Characteristics | Value (1,053 datapoints for n=35) |
|---|---|
| Steps | 35,604.0 (17,835.0–59,310.0) |
| Distance travelled (m) | 25,095.0 (11,635.0–42,002.5) |
| Floors climbed | 36.5 (6.0–72.2) |
| Time spent sitting (min) | 6,523.0 (5,190.8–8,532.2) |
| Energy expenditure total (kcal) | 15,370.0 (12,979.8–17,904.2) |
| Energy expenditure during activities (kcal) | 5,451.0 (1,871.5–8,543.8) |
| Low physical activity (min) | 1,220.5 (463.8–1,686.2) |
| Moderate physical activity (min) | 41.0 (0.0–127.2) |
| Moderate physical activity (min) grouped per WHO recommendation | |
| <150 min/week | 800/1,053 [76] |
| 150–300 min/week | 131/1,053 [12] |
| >300 min/week | 122/1,053 [12] |
| Vigorous physical activity (min) | 41.0 (0.0–127.2) |
| Vigorous physical activity (min) grouped per WHO recommendation | |
| <75 min/week | 636/1,024 [62] |
| 75–150 min/week | 164/1,024 [16] |
| >150 min/week | 224/1,024 [22] |
Values are given in numbers [%] for categorical or medians (interquartile ranges) for continuous variables accordingly. WHO, World Health Organization.
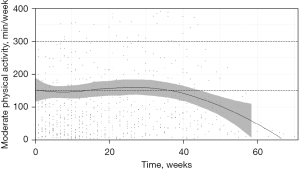
Table 4
| Variable | From week 0 to 20 | From week 20 to 40 | After week 40 | |||||
|---|---|---|---|---|---|---|---|---|
| Effect | P value | Effect | P value | Effect | P value | |||
| Sex† | ||||||||
| Male | 1.014 (1.000–1.027) | 0.054 | 1.018 (0.998–1.038) | 0.074 | 0.952 (0.926–0.979) | <0.001 | ||
| Female | 1.001 (0.977–1.027) | 0.908 | 1.025 (1.011–1.039) | <0.001 | 1.009 (0.995–1.023) | 0.202 | ||
| Relative MR (interaction term) | 0.988 (0.961–1.016) | 0.405 | 1.007 (0.983–1.031) | 0.580 | 1.060 (1.027–1.094) | <0.001 | ||
| Nutritional support before liver transplant† | ||||||||
| No | 1.009 (0.995–1.023) | 0.202 | 1.023 (1.003–1.042) | 0.024 | 0.970 (0.916–1.027) | 0.302 | ||
| Yes | 1.022 (0.995–1.050) | 0.106 | 1.011 (1.000–1.022) | 0.045 | 0.962 (0.950–0.974) | <0.001 | ||
| Relative MR (interaction term) | 1.013 (0.983–1.044) | 0.399 | 0.989 (0.967–1.011) | 0.320 | 0.991 (0.935–1.052) | 0.775 | ||
| Age (years)‡ | 1.000 (1.000–1.001) | 0.454 | 1.000 (0.998–1.001) | 0.546 | 0.997 (0.992–1.002) | 0.266 | ||
| Child-Pugh Score inclusion‡ | 1.001 (0.992–1.009) | 0.872 | 1.011 (0.994–1.029) | 0.195 | 0.983 (0.948–1.019) | 0.355 | ||
| Liver frailty Index at inclusion‡ | 0.998 (0.972–1.025) | 0.869 | 1.001 (0.985–1.017) | 0.899 | 1.021 (0.992–1.052) | 0.156 | ||
| SF36 total score at inclusion‡ | 0.998 (0.997–1.000) | 0.095 | 1.004 (0.999–1.009) | 0.105 | 1.013 (0.994–1.031) | 0.180 | ||
Analyses were done using a mixed-effects generalized linear model with a Gamma distribution and a log link. †, regression coefficient (95% CI); ‡, multiplicative change regression coefficient (95% CI). MR, mean ratio; CI, confidence interval.
Post-transplant outcome
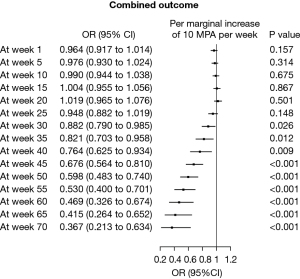
Events included in the composite clinical endpoint (including Re-LT, post-LT emergency re-hospitalisation or in-hospital complication during LT) were detected in 15 (42.9%) of patients. Figure 3A,3B display the temporal changes of MPA for the a priori chosen categorical factors sex and nutritional support for stratified outcome. Figure 4 depicts a univariate logistic regression with subsequent presentation in a Forest Plot to assess for significance. Significant risk of occurrence with regard to MPA (in steps of +10 min) occurred after week 30 [odds ratio (OR) 0.882, 95% CI: 0.790–0.985, P=0.026] and OR sequentially increased to 0.367 (95% CI: 0.213–0.634, P<0.001) at week 70 of listing (Figure 4). Dynamic longitudinal temporal stratification is displayed using LOESS regression in Figure 3 and Figures S1,S2. On crude univariate logistic regression analysis, nutritional support did not significantly reduce the occurrence of adverse clinical endpoints (OR 0.848, 95% CI: 0.179–3.740, P=0.829). Patients achieving WHO-recommended weekly MPA targets showed a lower risk of adverse composite clinical outcomes [endpoint: <150 min/week 350 (84.4%), 150–300 min/week 26 (6.3%), >300 min/week 39 (9.4%) vs. no endpoint: <150 min/week 450 (70.5%), 150–300 min/week 105 (16.5%), >300 min/week 83 (13.0%); P<0.001].
Discussion
Key findings, explanation and comparison
In our prospective study, we are able to show a clear association between pre-transplant decreased weekly moderate PA, as measured by a wristband activity tracker, and the increased rate of morbidity after LT. This was particularly apparent after a long time (30 weeks) on the waiting list. Importantly, patients achieving the WHO-recommended weekly MPA targets showed a lower risk of adverse composite clinical outcomes after LT. Our findings underline the importance of pre-transplant interventions aimed at improving the physical performance in an often frail and fragile patient population. This becomes even more important as the average age of the organ recipients is increasing and with it, the number of comorbidities the patients have. Achieving the aim of improving pre-transplant fitness within a structured pre-habilitation program, tailored to this patient population as is already recommended by several societies, will be essential to maintain high post-transplant success rates (26,31). Crespo et al. identified 4 assessment tools to detect patients suitable for Enhanced Recovery After Surgery (ERAS) protocols in the pre-transplantation period [Karnofsky performance status (KPS), LFI, abdominal muscle mass, cardiopulmonary exercise testing] (32). A recent systematic review on prehabilitation in patients awaiting LT did not detect an impact on adverse clinical outcomes including mortality due to sparse data (33). This lack of findings might also be confounded by not having identified the beneficial exercise intervention or prehabilitation program so far in these patients (34).
Our findings show that a decline in PA is in line with other identified decreasing PA measures (e.g., 6-minute walk, chair stands, hand grip) (4,35,36). De Smet et al. (16) and Berben et al. (37) have recently performed two separate meta-analyses on PA in the post-transplantation period, showing improved cardiorespiratory fitness measures and QoL. Subsequent limitations were discussed in detail by Thuluvath et al. (38). In a Korean study, declined physical performance (per the KPS) during the early post-transplantation period (one vs. six months post-transplant) was associated with increased mortality up to 5 years post-LT (15). Assessing the KPS or Rosow-Breslau Score are quite complex and time-consuming, while downloading data from a wrist-tracking device is not only quick but also objective, combing many different variables.
In our study, we were able to show a significant association between outcomes and decreased PA. Our data show that nutritional supplementation was associated with improved MPA between week 20 and 40 in patients awaiting LT, suggesting a benefit of this intervention. The clinical interpretation is challenging, as there is likely to be a selection bias in as much as that severe sarcopenic patients are typically those receiving nutritional support. Nutritional support may thus be considered a surrogate marker for the extent of the disease severity in waitlisted patients. The interpretation in this sense is comparable to the restrictions of the interpretation of the Model End-Stage Liver Disease in these frail patients.
Our cohort showed a difference in MPA over time, stratified according to the recipient sex, with an increase in MPA at inclusion (P=0.054 and P=0.074) in males (n=25) and a significant decrease (P<0.001) only after week 40 onwards. In females (n=10) on the other hand, there was a significant increase found solely between week 20 and 40 (P<0.001) of inclusion. A significant difference between sexes was identified after week 40 (P<0.001).
Interestingly, both patients with and without nutritional support showed a significant increase of MPA over time between week 20 and 40 after inclusion. After week 40, a significant decrease (P<0.001) of MPA over time in the group receiving nutritional support was noted possibly suggesting a “point of no return” of malnutrition in this very advanced phase of ESLD as was discussed by Dasarathy (39).
Strengths and limitations
While the majority of the available literature focuses on the post-transplantation period, we present data to support the use of health-prevention measures and implementation of an active pre-habilitation program in patients awaiting LT. This is of particular relevance in countries like Switzerland where patients spend long average times on the waiting list (40-44). The sex discrepancy of MPA over time in our cohort might be due to power, ascertainment bias or insufficient adherence regarding wearing of the tracking device or sex-related differences in underlying pathology and/or co-morbidities. Limitations include the inability to assess who was actually wearing the device and the small patient sample size. Resulting limitations are encountered in subgroup analyses. As such, which type of nutritional support might result in the most benefit for the patient in this setting, could not be assessed. In-depth analysis of adverse outcome endpoints (such as infections) was not feasible due to inadequate power due to the small sample size. The non-significance within the nutritional vs. no nutritional support group may be due to under-powering regarding this factor.
Moreover, a Hawthorne effect (45) or corresponding ascertainment bias cannot be ruled out, as no measurements were carried out prior to the study inclusion.
Implications and actions needed
The wristband activity tracking device or other wearable devices allow for a personalized approach, adapted to an individual’s possibilities and needs, serving as a telemedicine device to intervene in between the outpatient consultations. The latter aspects are particularly interesting for patients living far away from their transplant center, who are not seen on a regular basis. Home-based exercise has been shown to effectively improve sarcopenia (measured using the skeletal muscle index) and QoL (46) while increasing cardiopulmonary and aerobic capacity (47,48) in ESLD patients, with a wristband tracking device would allow for a closer follow-up (and intervention) in these patients.
The results of our study underline that intervention studies in the field of PA possibly combined with nutritional support are urgently needed to reduce morbidity and mortality before and after LT. Ultimately, pre-habilitation in combination with nutritional support might help stabilize or even reduce sarcopenia and frailty (49). Furthermore, involving patients in such a program will help to positively influence their sedentary life style as well as improving cardiorespiratory fitness (50) while decreasing postoperative morbidity and mortality. Influencing frailty and sarcopenia are essential, as they impact delisting and post-transplant outcomes (51).
Conclusions
Our findings show a significant association between decreased PA and adverse clinical outcome, especially after week 30 on the waiting list. Collectively, our findings support the implementation of a structured pre-habilitation program for patients awaiting LT.
Acknowledgments
We would like to acknowledge and thank our study nurses Olivier Kremo and Kathrin Husi as well as the transplant coordinators for helping us carry out the study. Moreover, we thank Odile Stadler from the clinical trial unit of the University of Bern for the statistical support. The wearable device was funded by the study center from internal funds.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-399/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-399/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-399/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-399/coif). C.B. received grant from Stiftung für Leberkrankheiten Bern. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local ethics committee (Kantonale Ethikkommission, Kanton Bern, BASEC ID 2019-00606). All patients included in the present study signed a specific informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Belarmino G, Gonzalez MC, Sala P, et al. Diagnosing Sarcopenia in Male Patients With Cirrhosis by Dual-Energy X-Ray Absorptiometry Estimates of Appendicular Skeletal Muscle Mass. JPEN J Parenter Enteral Nutr 2018;42:24-36. [Crossref] [PubMed]
- Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166-73, 173.e1.
- Giusto M, Lattanzi B, Albanese C, et al. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 2015;27:328-34. [Crossref] [PubMed]
- Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373-8. [Crossref] [PubMed]
- Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126-35. [Crossref] [PubMed]
- Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, et al. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122-39. [Crossref] [PubMed]
- Allen SL, Quinlan JI, Dhaliwal A, et al. Sarcopenia in chronic liver disease: mechanisms and countermeasures. Am J Physiol Gastrointest Liver Physiol 2021;320:G241-57. [Crossref] [PubMed]
- Dunn MA, Josbeno DA, Schmotzer AR, et al. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1324-32. [Crossref] [PubMed]
- Kumar R, Prakash SS, Priyadarshi RN, et al. Sarcopenia in Chronic Liver Disease: A Metabolic Perspective. J Clin Transl Hepatol 2022;10:1213-22. [Crossref] [PubMed]
- Kim G, Kang SH, Kim MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One 2017;12:e0186990. [Crossref] [PubMed]
- Kardashian A, Ge J, McCulloch CE, et al. Identifying an Optimal Liver Frailty Index Cutoff to Predict Waitlist Mortality in Liver Transplant Candidates. Hepatology 2021;73:1132-9. [Crossref] [PubMed]
- Tapper EB. Frailty and Outcomes After Liver Transplantation. Curr Transplant Rep 2019;6:1-6. [Crossref] [PubMed]
- Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant 2018;18:1986-94. [Crossref] [PubMed]
- Pieber K, Crevenna R, Nuhr MJ, et al. Aerobic capacity, muscle strength and health-related quality of life before and after orthotopic liver transplantation: preliminary data of an Austrian transplantation centre. J Rehabil Med 2006;38:322-8. [Crossref] [PubMed]
- Kim DG, Hwang S, Lee KW, et al. Physical Performance Decline During the Early Posttransplantation Period Affects Survival After Living Donor Liver Transplantation. Transplantation 2023;107:1545-53. [Crossref] [PubMed]
- De Smet S, O'Donoghue K, Lormans M, et al. Does Exercise Training Improve Physical Fitness and Health in Adult Liver Transplant Recipients? A Systematic Review and Meta-analysis. Transplantation 2023;107:e11-26. [Crossref] [PubMed]
- Meri C B, Solak S, Aydogdu N, et al. The comparison of endothelial function of moderate intensity interval exercise with continuous exercise in healthy men. Curr Res Physiol 2022;5:338-43. [Crossref] [PubMed]
- Barberan-Garcia A, Ubré M, Roca J, et al. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann Surg 2018;267:50-6. [Crossref] [PubMed]
- Jain SR, Kandarpa VL, Yaow CYL, et al. The Role and Effect of Multimodal Prehabilitation Before Major Abdominal Surgery: A Systemic Review and Meta-Analysis. World J Surg 2023;47:86-102. [Crossref] [PubMed]
- Chen J, Zhou R, Feng Y, et al. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct Target Ther 2022;7:383. [Crossref] [PubMed]
- Perry AS, Annis JS, Master H, et al. Association of Longitudinal Activity Measures and Diabetes Risk: An Analysis From the National Institutes of Health All of Us Research Program. J Clin Endocrinol Metab 2023;108:1101-9. [Crossref] [PubMed]
- Kandola A, Ashdown-Franks G, Hendrikse J, et al. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev 2019;107:525-39. [Crossref] [PubMed]
- Gloor S, Misirlic M, Frei-Lanter C, et al. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: results of a single-center, blinded, randomized controlled trial. Langenbecks Arch Surg 2022;407:897-907. [Crossref] [PubMed]
- O'Doherty AF, West M, Jack S, et al. Preoperative aerobic exercise training in elective intra-cavity surgery: a systematic review. Br J Anaesth 2013;110:679-89. [Crossref] [PubMed]
- WHO. Guidelines on physical activity and sedentary behaviour: at a glance. Vol. 1, 2020:1-17. Available online: https://www.who.int/publications/i/item/9789240015128
- Pollok JM, Tinguely P, Berenguer M, et al. Enhanced recovery for liver transplantation: recommendations from the 2022 International Liver Transplantation Society consensus conference. Lancet Gastroenterol Hepatol 2023;8:81-94. [Crossref] [PubMed]
- Functional Assessment in Liver Transplantation. Liver Frailty Index™. 2021. Available online: https://liverfrailtyindex.ucsf.edu
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Janaudis-Ferreira T, Mathur S, Deliva R, et al. Exercise for Solid Organ Transplant Candidates and Recipients: A Joint Position Statement of the Canadian Society of Transplantation and CAN-RESTORE. Transplantation 2019;103:e220-38. [Crossref] [PubMed]
- Crespo G, Hessheimer AJ, Armstrong MJ, et al. Which preoperative assessment modalities best identify patients who are suitable for enhanced recovery after liver transplantation? A systematic review of the literature and expert panel recommendations. Clin Transplant 2022;36:e14644. [Crossref] [PubMed]
- Jetten WD, Hogenbirk RNM, Van Meeteren NLU, et al. Physical Effects, Safety and Feasibility of Prehabilitation in Patients Awaiting Orthotopic Liver Transplantation, a Systematic Review. Transpl Int 2022;35:10330. [Crossref] [PubMed]
- Serper M, Jones LS, Clement T, et al. A randomized, controlled, prehabilitation intervention to maximize early recovery (PRIMER) in liver transplantation. Liver Transpl 2024;30:10-9. [Crossref] [PubMed]
- Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574-80. [Crossref] [PubMed]
- Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870-9. [Crossref] [PubMed]
- Berben L, Engberg SJ, Rossmeissl A, et al. Correlates and Outcomes of Low Physical Activity Posttransplant: A Systematic Review and Meta-Analysis. Transplantation 2019;103:679-88. [Crossref] [PubMed]
- Thuluvath AJ, Lai JC. Understanding Current Limitations to Exercise Interventions After Liver Transplantation. Transplantation 2023;107:e1-2. [Crossref] [PubMed]
- Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol 2016;32:159-65. [Crossref] [PubMed]
- Steck P, Immer F. Organspende: Lange Wartezeiten für Patientinnen und Patienten. Schweizerische Ärztezeitung 2022;103:589-92.
- De Geest S, Burkhalter H, Berben L, et al. The Swiss Transplant Cohort Study's framework for assessing lifelong psychosocial factors in solid-organ transplants. Prog Transplant 2013;23:235-46. [Crossref] [PubMed]
- Koller MT, van Delden C, Müller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol 2013;28:347-55. [Crossref] [PubMed]
- Eden J, Sousa Da Silva R, Cortes-Cerisuelo M, et al. Utilization of livers donated after circulatory death for transplantation - An international comparison. J Hepatol 2023;78:1007-16. [Crossref] [PubMed]
- Swisstransplant. Abb 4.13 - Wartezeit bis zur Transplantation. 2023. Available online: https://www.swisstransplant.org/de/organ-gewebespende/statistiken/jahreszahlen-1
- Demetriou C, Hu L, Smith TO, et al. Hawthorne effect on surgical studies. ANZ J Surg 2019;89:1567-76. [Crossref] [PubMed]
- Sirisunhirun P, Bandidniyamanon W, Jrerattakon Y, et al. Effect of a 12-week home-based exercise training program on aerobic capacity, muscle mass, liver and spleen stiffness, and quality of life in cirrhotic patients: a randomized controlled clinical trial. BMC Gastroenterol 2022;22:66. [Crossref] [PubMed]
- Román E, García-Galcerán C, Torrades T, et al. Effects of an Exercise Programme on Functional Capacity, Body Composition and Risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One 2016;11:e0151652. [Crossref] [PubMed]
- Chen HW, Ferrando A, White MG, et al. Home-Based Physical Activity and Diet Intervention to Improve Physical Function in Advanced Liver Disease: A Randomized Pilot Trial. Dig Dis Sci 2020;65:3350-9. [Crossref] [PubMed]
- Lin FP, Visina JM, Bloomer PM, et al. Prehabilitation-Driven Changes in Frailty Metrics Predict Mortality in Patients With Advanced Liver Disease. Am J Gastroenterol 2021;116:2105-17. [Crossref] [PubMed]
- Debette-Gratien M, Tabouret T, Antonini MT, et al. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation 2015;99:145-50. [Crossref] [PubMed]
- Ferreira AP, Machado MV. Impact of pretransplant frailty and sarcopenia on the post-transplant prognosis of patients with liver cirrhosis: a systematic review. Eur J Gastroenterol Hepatol 2021;33:e883-97. [Crossref] [PubMed]