Living donor liver transplantation for hepatocellular carcinoma achieves better outcomes
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common and the third causes of cancer-related mortalities. HCC is closely associated with chronic liver disease and as many as 80% of cases occur in cirrhotic livers. Although liver resection and local ablation are regarded as potentially curative treatments, the limited functional reserve of the liver restricts their application and there is a high chance of recurrence in the liver remnant (1). Liver transplantation (LT) is the only treatment that offers a chance of cure for the tumor and the underlying cirrhosis by complete extirpation of both. The outcome of LT for early HCC is encouraging, but the limitation of organ supply remains the main issue (2). In Asia, living donor LT (LDLT) has been emerging as the solution of organ shortage to treat HCC. At Kaohsiung Chang Gung Memorial Hospital, the first case of LDLT was performed for a pediatric patient with biliary atresia in 1994, and progressed to adult LDLT in 1999 (3). In the high endemicity of hepatitis B and C (HBV and HCV), HCC has become the most indication for LT. In this review, we summarize the experience of treating HCC in LDLT and current selection criteria, adjusted by our previous experience. Finally, we propose an algorithm of patient selection for HCC in LDLT to achieve better outcomes.
LDLT in Taiwan
The success of LT worldwide has brought increased demand for the liver graft. Western and Asian countries have coped differently with the problems of the shortages in organ donation. The great advances in the field of LDLT have been dictated by the needs and the norms of Asian society. In Taiwan, although the society endeavors to improve rate of deceased organ donation, deceased donor LT (DDLT) just increased slightly; in contrast, LDLT increased over 3-fold cases at the same period from 2006 to 2010 (4).
LDLT for HCC
The incidence of liver cancer in Taiwan deceased to the third place from the second since 2012 but liver cancer-related mortality still remained in the second among all cancer in Taiwan (5). Every year around 800 new patients of HCC were registered at our hospital. LDLT for the patient with HCC was firstly performed in 2000. Since then, the number of patients with HCC increased year upon year; almost 50% recipients of LDLT currently had a history of HCC (Figure 1).

Philosophy of LDLT
There are still remaining a lot of debates about selection criteria for patients with HCC between DDLT and LDLT. In LDLT, LDs could be considered as recipient personal gifts. Without sharing public resources, LDLT could be used in patients with extended criteria, which is not accepted in waiting list of DDLT. Predictably, extended criteria lead to higher recurrence rate and consequent worse long-term survival (6). In contrast, owing to taking the risk of LD, we are obligated to adopt strict selection criteria for HCC patients and optimize the pre-transplant conditions to ensure a high disease-free survival similar to those without HCC, even better than DDLT. Basically there is no difference in the management strategy between DDLD and LDLT after transplant. LDLT definite provides some advantages during transplant such as shortening waiting time, consistent high quality of grafts. In addition, LDLT importantly offers the opportunity of optimizing the time of transplant and pre-transplant managements to ensure as high as possible outcomes. To achieve better outcomes of treating HCC by LDLT relies on low surgical mortality and optimal selection criteria.
Considerations of primary vs. salvage LDLT
With the advances of surgical techniques, primary LDLT for HCC have been a standard procedure and performed with low surgical mortality and over 90% 5-year survival rate at our center (7,8). Resectable HCC with preserved liver function present a therapeutic dilemma. Resection is first-line curative treatment for HCC with low cost and surgical mortality. Furthermore, the pathologic findings of complete resection provide the definite histology and differentiation of HCC and complete evaluation of vascular invasion, especially for the tumors adjacent to major vascular structure. Salvage LT is preserved until tumor recurrence or deteriorated liver function (9). However, the sequent portal hypertension and collaterals combined with dense adhesion from the previous resection could lead to more blood loss and surgical difficulty during the following LT. In addition, the patients have the risks of becoming untransplantable due to the presentation of recurrent tumors beyond criteria (10). Therefore, facing the dilemma, the results of salvage LDLT play a pivotal role in justifying the decision of resection as the first choice following by LDLT if necessary.
A recent meta-analysis indicated that salvage LT has a similar long-term survival rate with comparable surgical complications, compared to that of primary LT (11). However, the majority of salvage LT patients underwent DDLT. In LDLT, it is worth noting that the techniques are more challenging since vascular and biliary pedicles are smaller and shorter in salvage LDLT. Dense adhesion from previous surgery combined with collateral vein could lead to massive bleeding which requires damage control measures and even staged biliary reconstruction in another operation. In our first 100 salvage LDLT study, the hospital mortality decrease from 8% of first 50 cases to 2% of latter 50 cases, which was similar to primary LDLT (12). With this excellent surgical outcomes of salvage LDLT, resection is considered first-line treatment for HCC with preserved liver function to obtain histology, especially in cases of suspicious of unfavorable histology and major vascular invasion.
Selection criteria in within UCSF era
Selection criteria
In the early period of developing LT, there were extensive debates in the selection criteria for LT in HCC patients because of the disappointing survival without appropriate selection. However, we learned lessons from these experiences. First, it is necessary to have more intensive screening of high risk groups; second, larger-sized tumor does not definitely indicate poorer outcome; third, recurrence rate is higher and survival is poorer in a certain histological subtypes of HCC (13,14). The selection criteria of LT directly influence the outcome and currently we have various selection systems. It’s just like what “Metroticket” study group demonstrated, the longer the trip, the higher the price. Currently, the tumor size and the number are the main tumor characteristics used to determine eligibility for LT (15). Since the publication of the Milan criteria in 1996, transplantation has been recognized as the best treatment for patients with small non resectable HCC (2). The 5-year survival was between 70% and 90% if patient selection is strictly adhered to Milan criteria. Several studies however have now demonstrated that the Milan criteria are too restrictive and that favorable outcomes can be achieved following more liberal selection policies. The group at the UCSF, was the first to propose expanded criteria. The UCSF criteria was initially based on explant pathology and demonstrated a non-inferior result, 75% 5-year survival rate, comparing with Milan criteria (16).
Results of downstaging
In our early experience, the selection criteria of LT for HCC patients was restricted to Milan criteria by national insurance policy. The HCC patients presented with tumors beyond criteria should be downstaged to fit criteria. Our initial results of 35 patients were encouraging as recipients whose tumors had been downstaged into Milan criteria had not have recurrence (8). The 5-year survival rate was 90%; importantly to date those patients are still disease-free after follow-up of 10 years. Since July 2006, the criteria were extended to UCSF criteria for HCC patients. In our experience of 161 HCC patients, 51 (31.6%) were successfully downstaged to fit UCSF criteria. The overall 1- and 5-year survival rates were comparable; 94.1% versus 92.7% and 83.7% versus 78.9% of downstaged versus non-downstaged, respectively. The survival rates were similar between different pretransplant downstage procedures (17).
Risk factors for recurrence after transplantation
In addition of morphological selection, adequate patient selection should be based on tumor biology assessed via serum or pathological parameters and functional/radiological features of tumors.
Alpha-fetoprotein (AFP)
AFP is an attractive prognostic maker that has been studied extensively in HCC. AFP may be a surrogate for vascular invasion and predict HCC recurrence. There are numerous existing studies in the literature to suggest a clear prognostic value for pretransplant AFP levels, although there is no validated cut-off value which can be standardized across patient groups. Recent systemic reviews showed better outcomes for the HCC patients with lower (<1,000 ng/mL) pretransplant AFP levels. Furthermore, high pretransplant AFP levels (>1,000 ng/mL) were associated with vascular invasion and poor tumor differentiation (18). In USA, AFP level >1,000 ng/mL was considered as an exclusion criterion for LT within the Milan criteria unless the AFP level decreases to <500 ng/mL after downstaging treatments, in hope of improving posttransplant outcomes (19). There are several other cuff-off values of AFP, which are used to predict post-transplant HCC recurrence and survival; no consensus is however achieved since the criteria and outcomes are widely varied. But the tentative conclusion could be made that the lower AFP level of selection criteria provides the better outcomes.
In addition of static pretransplant AFP value, dynamic slope of serial AFP measurements would be a better predictor of survival and recurrence (20). In our unpublished data of over 400 HCC patients, AFP level (>70 ng/mL) is associated with higher recurrence rate. The patients with high AFP are not eligible for LDLT until AFP decreases to low level after downstaging.
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan
The glucose metabolism of HCC is related to tumor grade and aggressive biological properties such as differentiation/microvascular invasion and can be assessed with F-18 FDG-PET (21,22). Therefore, the FDG avidity of HCC seems to be a potential biomarker for pretransplant evaluation of the risk of HCC recurrence after LT. There is growing evidence that FDG-PET can identify patients at risk for the recurrence of HCC after LT (22-24). Our recent study in 147 patients with pretransplant FDG-PET exam showed the 5-year recurrent free survivals were 94% in the low risk group (within USCF criteria and FDG-negative), 75.8% in the intermediate risk group [beyond UCSF criteria and FDG-negative, or low standard uptake value (SUV)]. Importantly, the ratio of tumor to normal-liver SUV plays an essential role to predict tumor recurrence. In the high risk group (FDG-positive with high SUV), the 1-, 3-, 5-year recurrence free survivals significantly decreased to 44.4%, 29.6%, and 29.6% (25).
Unfavorable histology
On the other hand, even restricting selection criteria of LT for HCC patients within the Milan criteria fails in completely ruling out HCC recurrence since some small HCCs have aggressive features such as poorly differentiated grade or vascular invasion (26). Poorly differentiated grade of tumor has been considered an independent predictor factor for post-LT HCC recurrence (27). Some rare histological type of HCC carries a poor prognosis. Two cases of sarcomatoid HCC with normal AFP and within Milan criteria developed HCC recurrence within 6 months after LDLT in our series. Combined HCC and cholangiocarcinoma (CC) is another poor prognostic predictor for HCC recurrence. The results of LT for combined HCC and CC is far inferior to that for HCC; resection may be considered the best therapeutic approach for such patients (28). Similarly, in our experience of 12 patients with combined HCC and CC, the 5-year survival is around 50%, compatible with the reports from other groups. Since the diagnosis based on histology would allow to improve outcomes further, histological assessment by liver biopsy or resection may be essential to rule out poorly differentiated tumors, sarcomatoid and combined HCC and CC.
Revised criteria of downstaging
With accumulation of experience, the inclusion and exclusion criteria of downstaging at our center have been refined. On top of UCSF criteria, the inclusion criteria include modifiable biological makers of high AFP and high PET-SUV ratio according to our previous study. During the initial evaluation, the exclusion criteria by the image study contain major vascular invasion, massive infiltrative type, rupture (or by history) and distant metastasis.
There is currently no well-defined upper limit in terms of size and number of tumor as eligibility for downstage criteria. Several entry criteria for downstaging have been proposed but the meta-analysis meet the difficulty because of the consideration of selection criteria for HCC, organ availability and the setting of DDLT or LDLT. In a recent national conference in US, the working group proposed inclusion criteria including a single tumor ≤8 cm or two to three tumors, each ≤5 cm, with a total tumor diameter ≤8 cm (29). The upper limit seems to be extended regarding the size and number of the tumor. Especially, several small particles have been invented to create TACE with drug-eluting beads, which is a promising alternative to conventional drugs. The treatment response showed significantly better and delayed disease progression compared with conventional TACE (30). It sounds reasonable to downstage each patient if the tumors present without absolute contraindications.
The minimal observation period from successful downstaging to LT is required to be a selection of HCC with a favorable biology. The optimal length of this period of observation for tumor biology is unknown (29). In our protocol, this observation period is 3 months but we try to reduce the observation time to test the hypothesis whether reduction of tumor burden decreases posttransplant recurrence, not from tumor biology selection, mirrored by good response to treatment.
Necrotizing therapy
Explanted liver pathology
Local regional therapy (LRT) has mostly been used to mitigate the risk of tumor progression and dropout on waiting list by achieving pathologic tumor necrosis. The complete pathologic response (cPR) was around 27% to 57% of patients after TACE and 47% to 75% after radiofrequency ablation (RFA) (31,32). Recent report demonstrated that cPR reduced the recurrence of HCC after transplant and almost reduced to below 3% (33). Our unpublished data of 453 patients also showed similar results; the 5-year disease-free survival of patients is 98% in cases of no viable tumor in explanted liver, significantly better than those with viable tumor (89%).
Necrotizing therapy
In DDLT setting, pretransplant LRT is necessary to decrease wait list dropout, particularly for patients expected to wait longer than 3 to 6 months for LT and those with risk factors such as a focal HCC >3 cm in greatest diameter or multiple HCCs. In USA, 31% to 65% of recipients received LRT before transplant on the different allocation regions (29). Unlike DDLT, additional LRT is performed in order to prevent tumor progression on waitlist. Taking the advantage of donor availability in LDLT, additional LRT is performed to achieve complete tumor necrosis before transplant. It is so-call necrotizing therapy to minimize the possibility of tumor recurrence even the tumors present within criteria before transplant.
Summary
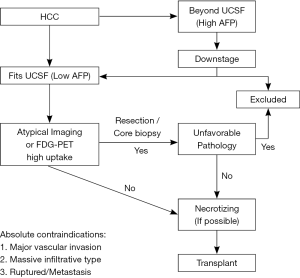
Based on the aforementioned experience, the selection criteria of patients with HCC is illustrated in Figure 2. The management of HCC should be relied on a stepwise approach that incorporates morphological and biological criteria of the tumor. This approach aims at tailoring the best treatment plan for each individual patient. The absolute contraindications for transplant includes major vascular invasion, massive infiltrative type, ruptured HCC and distant metastasis. Firstly, the tumor should be fit not only UCSF criteria but also the level of AFP, otherwise the downstaging procedures are administered to fit UCSF criteria with low AFP. Thereafter, since the diagnosis of HCC is based mainly on a clinical context associated with typical imaging criteria., atypical imaging or positive PET with high SUV necessitates tissue proof by a core biopsy or resection to exclude the unfavorable tissue types such as poor differentiation, combined with CC and sarcomatoid type HCC. In addition, if the FDG-PET yields negative result or positive result with low SUV, LDLT is considered. If the FDG-PET yields high SUVmax (>5), tumor downstaging to reduce the tumor burden is required, either by liver resection or LRT. LDLT is not considered until repeated FDG-PET yields low SUV. In the final step, to minimize the recurrence rate of HCC after transplant, additional LRT is recommended if possible before transplant to necrotize tumors. By taking the advantage of donor availability, we believe that multiple steps of selections and optimal managements provide the better short-term and long-term outcomes by minimizing the opportunities of HCC recurrence.
Acknowledgements
Funding: This work was supported by grants from Health and Welfare Surcharge of Tobacco Products, Ministry of Health and Welfare, Taiwan (MOHW103-TD-B-111-07, MOHW104-TDU-B-212-124-004, MOHW105-TDU-B-212-134006 to CL Chen).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Chen CL, Concejero A, Wang CC, et al. Living donor liver transplantation for biliary atresia: a single-center experience with first 100 cases. Am J Transplant 2006;6:2672-9. [Crossref] [PubMed]
- Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol 2013;10:746-51. [Crossref] [PubMed]
- Ministry of Health and Welfare, 2015. Available online: http://www.mohw.gov.tw/news
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Chen CL, Fan ST, Lee SG, et al. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation 2003;75:S6-11. [Crossref] [PubMed]
- Concejero A, Chen CL, Wang CC, et al. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation 2008;85:398-406. [PubMed]
- Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology 2000;31:899-906. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- Hu Z, Wang W, Li Z, Ye S, et al. Recipient outcomes of salvage liver transplantation versus primary liver transplantation: a systematic review and meta-analysis. Liver Transpl 2012;18:1316-23. [Crossref] [PubMed]
- Lin C, Lin T, Wang C, et al. Does salvage living donor liver transplantation carry higher morbidities and mortalities? Transplantation 2015;99:93. (abstact).
- Iwatsuki S, Gordon RD, Shaw BW Jr, et al. Role of liver transplantation in cancer therapy. Ann Surg 1985;202:401-7. [Crossref] [PubMed]
- O'Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg 1988;207:373-9. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Yu CY, Ou HY, Huang TL, et al. Hepatocellular carcinoma downstaging in liver transplantation. Transplant Proc 2012;44:412-4. [Crossref] [PubMed]
- Hakeem AR, Young RS, Marangoni G, et al. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:987-99. [PubMed]
- Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. [Crossref] [PubMed]
- Han K, Tzimas GN, Barkun JS, et al. Preoperative alpha-fetoprotein slope is predictive of hepatocellular carcinoma recurrence after liver transplantation. Can J Gastroenterol 2007;21:39-45. [Crossref] [PubMed]
- Kitamura K, Hatano E, Higashi T, et al. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol 2011;55:846-57. [Crossref] [PubMed]
- Cheung TT, Chan SC, Ho CL, et al. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl 2011;17:1218-25. [Crossref] [PubMed]
- Kornberg A, Freesmeyer M, Bärthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592-600. [Crossref] [PubMed]
- Asman Y, Evenson AR, Even-Sapir E, et al. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl 2015;21:572-80. [Crossref] [PubMed]
- Hsu CC, Chen CL, Wang CC, et al. Combination of FDG-PET and UCSF Criteria for Predicting HCC Recurrence After Living Donor Liver Transplantation. Transplantation 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Mazzaferro V, Chun YS, Poon RT, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 2008;15:1001-7. [Crossref] [PubMed]
- Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502-9; discussion 509-11. [Crossref] [PubMed]
- Groeschl RT, Turaga KK, Gamblin TC. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J Surg Oncol 2013;107:608-12. [Crossref] [PubMed]
- Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262-78. [Crossref] [PubMed]
- Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012;57:1244-50. [Crossref] [PubMed]
- N'Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475-83. [Crossref] [PubMed]
- Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol 2015;63:83-92. [Crossref] [PubMed]
- Ravaioli M, Grazi GL, Ercolani G, et al. Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation 2004;78:1780-6. [Crossref] [PubMed]


