Living donor liver transplantation for hepatocellular carcinoma in Seoul National University
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequently diagnosed cancer worldwide and the third leading cause of cancer-related death in the world (1-3). The incidence of HCC varies according to geographic location due to various geographic exposure to hepatitis viruses. Asia, in that sense, is considered a high-incidence region with high frequency of hepatitis B virus (4). Liver transplantation is theoretically the best oncologic treatment for HCC by removing the tumor with the widest margin and curing the background cirrhosis. However, the initial results following liver transplantation for HCC in the late 1980s were disappointing with high tumor recurrence and poor 5-year survival deemed to be less than 40% (5-7). This led to Mazzaferro and colleagues suggesting a restrictive selection criteria, also known as the Milan criteria (8). The Milan criteria limited liver transplantation to patients with early HCC which is defined by a solitary tumor not exceeding 5 cm or no more than three tumors, each measuring less than 3 cm without major vessel invasion or extrahepatic tumor spread. Recently, the University of California at San Francisco (UCSF) proposed an extended criteria, which limits the selection to solitary tumor up to 6.5 cm or a maximum of 3 tumor nodules each up to 4.5 cm, and a total tumor diameter not exceeding 8 cm (9). Many centers adopted the Milan or UCSF criteria as a selection criteria in deciding suitable candidates with HCC for liver transplantation.
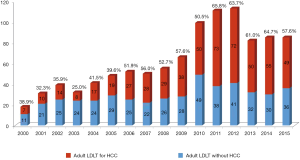
Similarly to Western countries, there is still a shortage of deceased organ donation in Asian countries (4). Additionally, the incidence of HCC is higher than Western countries due to a higher frequency of viral hepatitis. To overcome this shortage of supply, living donor liver transplantation (LDLT) has been developed and accepted as an alternative to deceased donor liver transplantation (DDLT) (Figure 1). Unlike DDLT, a potential liver graft is not a public resource but dedicated to a specific recipient in LDLT (10). Therefore, the criteria for HCC in LDLT are not as constrained by societal beneficence.
In this article, we introduce our experience and current outcomes of LDLT for HCC focusing on the application of our expanded selection criteria.
Methods
From January 2000 to December 2015, 546 adult patients underwent LDLT and had pathologically confirmed HCC at Seoul National University Hospital (SNUH). Of these, 14 patients with mixed tumors containing HCC and cholangiocarcinoma were excluded. As a result, 532 patients were included in this study. A retrospective review of medical records were performed and identified 532 patients with follow up until December 2015. In this study, the Milan criteria was determined on the basis of explant pathology.
Pre-transplantation evaluation
Computed tomography (CT), enhanced magnetic resonance imaging (MRI), and positron emission tomography (PET) were routinely checked for pre-transplantation HCC workup. In this study, we defined an iso-metabolic lesion as a PET negative and a mild or strong hypermetabolic lesion as a PET positive. We also evaluated tumor markers including alpha-fetoprotein (AFP) and protein induced by vitamin K absence-II (PIVKAII). We did not select the patients for transplantation based only on size and number and we expanded the criteria based on AFP, PIVKAII and PET positivity, according to our standard clinical practice. Our absolute contraindication was the patients with extrahepatic metastasis.
Post-transplantation management and follow-up
After transplantation, maintenance immunosuppression was based on triple regimen, which includes tacrolimus, mycophenolate mofetil (MMF), and steroid. For far advanced HCC (HCC larger than 10 cm or more than 10 numbers or with macrovascular invasion), we considered using mammalian target of rapamycin (mTOR) inhibitor-based immunosuppression.
Dynamic liver CT or MRI, chest CT, and bone scan including serum tumor markers were checked every 3 to 6 months for the first one and a half year depending on the risk factors such as high AFP, microvascular invasion, and high histological grade, and every 6 months to 1 year thereafter (11,12). PET was also performed for patients with a high-risk of recurrence, such as patients who did not meet the Milan criteria, and patients with increasing tumor markers without abnormal liver function.
Statistical analysis
Statistical analysis was performed using the SPSS software (version 22; SPSS, Chicago, IL, USA). Results are expressed as the median and range. Disease-free survival rate (DFSR) and survival rate (SR) were estimated with the Kaplan-Meier method and compared using a log-rank test. A probability (P) value less than 0.05 was considered significant.
Ethics statement
The institutional review board of SNUH approved this study (IRB No. 1604-051-753). The board exempted informed consent for this retrospective study of prospectively collected data.
Results
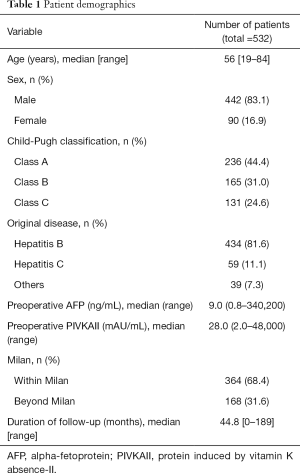
The median age was 56 years (range, 19–84 years). In total, 442 patients (83.1%) were male and 90 (16.9%) were female. Of the total, 236 patients (44.4%) were Child A, 165 (31.0%) were Child B, and 131 (24.6%) were Child C. Four hundred and thirty-four patients (51.6%) had hepatitis B as original disease and 59 (11.1%) had hepatitis C. The median preoperative AFP level was 9.0 (range, 0.8–340,200) and PIVKAII level was 28.0 (range, 2.0–48,000). Three hundred and sixty-four patients (68.4%) were within Milan criteria based on explant pathology and 168 (31.6%) were beyond Milan criteria. Microvascular invasion was noted in 100 patients (18.8%). The median follow-up period was 44.8 months (range, 0–189 months) (Table 1).
Full table
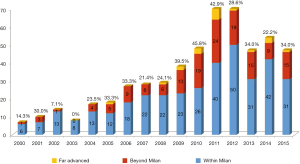
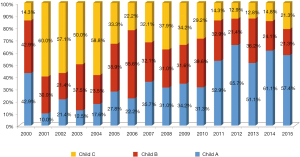
Annual proportion of HCC patients beyond Milan criteria is shown in Figure 2. LDLT patients beyond Milan criteria including far advanced HCC have recently accounted for about 30% to 40% of total LDLT for HCC. Annual proportion of HCC patients according to Child-Pugh classification is shown in Figure 3. The proportion of Child A patients has increased to 60% recently.
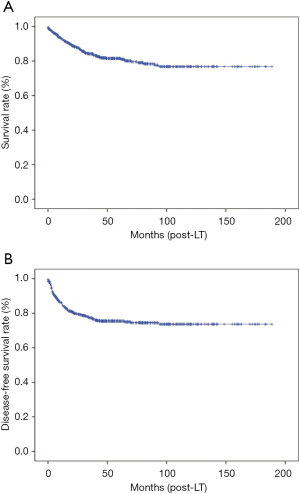
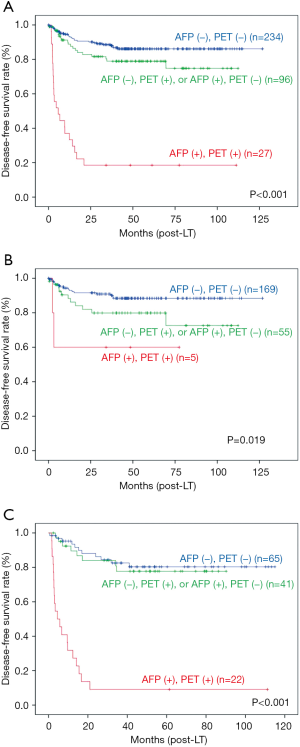
The 1- and 5-year overall SRs of the total 532 patients were 92.8% and 81.5% respectively. The 1- and 5-year DFSRs were 85.0% and 75.5% respectively (Figure 4). The risk stratification using SNUH criteria of tumor recurrence was performed according to the combination of two factors; low risk group [AFP <200 ng/mL, PET (−)], intermediate risk group [AFP >200 ng/mL, PET (−) or AFP <200 ng/mL, PET (+)] and high risk group [AFP >200 ng/mL, PET (+)] (13). The 5-year DFSR of low risk group was 86.1%, intermediate risk group was 79.0%, and high risk group was 18.5% (P<0.001). Within Milan criteria, the 5-year DFSR of low risk group was 88.4%, intermediate risk group was 79.9%, and high risk group was 60.0% (P=0.016). Beyond Milan criteria, the 5-year DFSR of low, intermediate, and high risk group was 80.3%, 77.7%, and 9.1%, respectively (P<0.001) (Figure 5).
Discussion
The optimal selection criterion of HCC patients in LDLT is controversial. Our data demonstrates a survival advantage for patients beyond traditional selection criteria. In our data more than 30% of our patients are beyond Milan at the time of transplant, this illustrates our willingness to expand recipient HCC criteria (Figure 2). We found a subset of patients who do not meet Milan criteria to have good prognosis after LDLT. According to our previous study, patients without vascular invasion and with preoperative AFP less than 400 ng/mL have acceptable prognosis following LDLT (11). We also reported that LDLT showed worse outcomes than DDLT among patients within the UCSF criteria when having poor tumor biology (high AFP, microvascular invasion, high histological grade). This may be due to different clinical characteristics of LDLT such as a fast-track effect and graft regeneration (12). Based on these results, conventional criteria from DDLT based analysis, such as Milan criteria and UCSF criteria, focusing on tumor number and size are not similarly effective to stratify survival in LDLT. We no longer use these conventional criteria as an exclusion criteria for LDLT at our center.
Primary resection is still the first treatment option in early HCC (14,15). However, primary resection has several limitations including limit by hepatic functional reserve, inability to correct background liver cirrhosis, and high intrahepatic recurrence rate. In Korea, 70% to 80% of HCC patients have chronic hepatitis B (16). In our study, hepatitis B patients have accounted for 81.6% of recipients. LDLT addresses both the tumor and the background liver cirrhosis. Additionally, LDLT is not limited by recipient hepatic functional reserve. However, LDLT has several limitations such as risk to the live donor, high cost, lifelong immunosuppression, and availability of a suitable voluntary donor. Similar to other studies, DFSR was better in LDLT patients than in resection patients, although resection patients show almost the same or slightly lower long-term overall SR in our previous analysis (17). As a result, primary resection and salvage LDLT after recurrence has been considered to be a feasible and rational strategy. However, the salvage liver transplantation should be restricted to patients with favorable oncological factors such as no microscopic vascular invasion, no satellite nodules, tumor size <3 cm, and well differentiated tumors (18). Furthermore, according to previous studies, half of the primarily resected patients had recurrence within 3 years and 20% to 75% had recurrence beyond Milan criteria preventing these patients from their chance of getting salvage transplantation (19,20). There are some patients with early HCC who strongly want LDLT as a primary treatment. Without clear criteria predicting no-recurrence or transplantable-recurrence after primary resection so far, we selectively accept primary LDLT in these patients with good liver function, especially in the following situations: (I) relatively young patients with strong family support; or (II) high chance of recurrence after resection, such as multiple dysplastic nodules in the background liver (10).
Recently, we have modified our selection criteria to incorporate biologic markers to identify HCC patients safe for LDLT. These biologic markers include AFP, PIVKAII, and PET positivity (11,21,22). The combination of the serum AFP level and PET predicted outcomes better than using Milan criteria (13). Also, in our study also, overall SR and DFSR showed significant difference according to PET positivity and AFP level cut-off value of 200 ng/mL (P<0.001). Patients with all three positive biologic factors are considered as relative contraindication regardless of Milan criteria. We developed a new scoring model using PIVKAII and AFP to refine prognostication (23). With this score less than 314.8 and without extrahepatic metastasis, HCC patients beyond Milan criteria are potential candidates for LDLT (23).
The primary concern in LDLT is donor safety, some reports document associated mortality and morbidity for the healthy donor were as high as 0.3% and 2%, respectively (24). Recipient benefit must take into account donor risk. Some centers, especially Asian centers where LDLT is a needed alternative to DDLT, consider 5-year SR of 50–60% to be acceptable (10,25). In more than 1,000 LDLT cases, we have not experienced any donor irreversible disability or mortality (26,27). The donor should be well informed and be willing to donate without coercion. Our protocol includes a multidisciplinary assessment of the donor by a psychologist, medical social worker, and a transplant coordinator.
Twenty-nine patients with advanced HCC underwent LDLT at our center from January 2003 to February 2013. As expected, the post-transplant outcomes of far advanced HCC patients were poor (3-year DFSR was 28.4%). However, patients with macrovascular invasion with AFP ≤200 ng/mL had excellent overall SR and DFSR after LDLT (3-year DFSR 87.5% and 65.6%) (27). Furthermore, recurrence does not mean death since the survival after recurrence differs case by case. Some patients have a good prognosis with appropriate treatment (28). In our center, we have expanded our indication to recipients with advanced HCC (HCC larger than 10 cm or more than 10 numbers or with macrovascular invasion). Based on our experiences, we are expanding the criteria selectively, including patients with macrovascular invasion, if the tumor biology is favorable and there are no other effective treatment options.
HCC recurrence after transplant results in significantly diminished survival. Immunosuppressant therapy required for liver transplantation may potentiate tumor recurrence (27). The prognosis after HCC recurrence depends on the time to recurrence and site of recurrence. However, some patients with recurrence can have a good prognosis, and the appropriate treatment can prolong their survival (28). Early detection and treatment may improve the long-term prognosis in HCC recurrence due to rapid tumor progression under the immunocompromised state (29). Our surveillance protocol relies on CT scans of the chest and abdomen, bone scans, and AFP. These tools evaluate extra-hepatic recurrence as well as intra-hepatic recurrence. When possible, surgery is considered for the recurrent disease as it has been associated with a better outcome (28,29).
Furthermore, we are investigating the role of modifying our immunosuppressant regimen to prevent HCC recurrence after transplant. There is mounting evidence to suggest that mTOR has direct anticarcinogenic effects and may reduce HCC recurrence rates (30). mTOR inhibitor based immunosuppression may delay tumor recurrence by suppressing cancer cell proliferation and downregulating vascular endothelial growth factor production (31). There are reports that converting to mTOR inhibitor based immunosuppression from tacrolimus can be helpful even after the recurrence (32). Furthermore, we have reported that combination of sirolimus and MMF showed antiproliferative effect consistently in vivo as well as in vitro (33). Due to the potential synergistic anticancer effect, mTOR inhibitor with MMF combination therapy is used for high risk of recurrence patients who underwent LDLT for HCC in our center.
There are some limitations to our study. It was a retrospective study and we were obligated to rely on the completeness of the medical records for our analysis. Our result may not be generalized to DDLT because the patients who underwent only LDLT, not DDLT, were included.
In conclusion, our data and experience suggest that a continued paradigm shift from conventional size based criteria to a biologic marker based criteria based on AFP, PIVKA II, and PET positivity when evaluating candidates with HCC for LDLT. Based on this new biological criteria and modified immunosuppression strategy, we are expanding the criteria from early HCC to advanced HCC. Extrahepatic metastasis is currently our absolute contraindication for LDLT.
Acknowledgements
All authors thank Dr. Stephen H. Gray for English editing and proofreading of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of SNUH (No. 1604-051-753) and written informed consent was obtained from all patients.
References
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003;139:817-23. [Crossref] [PubMed]
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33-64, 1.
- de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist 2007;12:1321-31. [Crossref] [PubMed]
- Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg 1988;12:39-47. [Crossref] [PubMed]
- Iwatsuki S, Gordon RD, Shaw BW Jr, et al. Role of liver transplantation in cancer therapy. Ann Surg 1985;202:401-7. [Crossref] [PubMed]
- O'Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg 1988;207:373-9. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Lee KW, Yi NJ, Suh KS. Section 5. Further expanding the criteria for HCC in living donor liver transplantation: when not to transplant: SNUH experience. Transplantation 2014;97 Suppl 8:S20-3. [Crossref] [PubMed]
- Suh KS, Cho EH, Lee HW, et al. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis 2007;25:329-33. [Crossref] [PubMed]
- Park MS, Lee KW, Suh SW, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014;97:71-7. [Crossref] [PubMed]
- Hong G, Suh KS, Suh S, et al. Alpha-fetoprotein and 18F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol 2016;64:852-9. [Crossref] [PubMed]
- Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl 2003;9:513-20. [Crossref] [PubMed]
- Margarit C, Escartín A, Castells L, et al. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl 2005;11:1242-51. [Crossref] [PubMed]
- Huh K, Choi SY, Whang YS, et al. Prevalence of viral hepatitis markers in Korean patients with hepatocellular carcinoma. J Korean Med Sci 1998;13:306-10. [Crossref] [PubMed]
- Yi NJ, Suh KS, Kim T, et al. Current role of surgery in treatment of early stage hepatocellular carcinoma: resection versus liver transplantation. Oncology 2008;75 Suppl 1:124-8. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 2003;238:508-18; discussion 518-9. [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-82. [Crossref] [PubMed]
- Yang SH, Suh KS, Lee HW, et al. A revised scoring system utilizing serum alphafetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery 2007;141:598-609. [Crossref] [PubMed]
- Lee JW, Paeng JC, Kang KW, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med 2009;50:682-7. [Crossref] [PubMed]
- Lee JH, Cho Y, Kim HY, et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann Surg 2016;263:842-50. [Crossref] [PubMed]
- Cheah YL, Simpson MA, Pomposelli JJ, et al. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl 2013;19:499-506. [Crossref] [PubMed]
- Lee HW, Suh KS. Expansion of the criteria for living donor liver transplantation for hepatocellular carcinoma. Curr Opin Organ Transplant 2016;21:231-7. [Crossref] [PubMed]
- Suh KS, Suh SW, Lee JM, et al. Recent advancements in and views on the donor operation in living donor liver transplantation: a single-center study of 886 patients over 13 years. Liver Transpl 2015;21:329-38. [Crossref] [PubMed]
- Suh KS, Lee HW. Liver transplantation for advanced hepatocellular carcinoma: how far can we go? Hepatic Oncol 2015;2:19-28. [Crossref]
- Shin WY, Suh KS, Lee HW, et al. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2010;16:678-84. [Crossref] [PubMed]
- Park MS, Lee KW, Yi NJ, et al. Optimal tailored screening protocol after living donor liver transplantation for hepatocellular carcinoma. J Korean Med Sci 2014;29:1360-6. [Crossref] [PubMed]
- Säemann MD, Haidinger M, Hecking M, et al. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant 2009;9:2655-61. [Crossref] [PubMed]
- Guba M, Graeb C, Jauch KW, et al. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004;77:1777-82. [Crossref] [PubMed]
- Vivarelli M, Dazzi A, Zanello M, et al. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation 2010;89:227-31. [Crossref] [PubMed]
- Lee KW, Seo YD, Oh SC, et al. What is the best immunosuppressant combination in terms of antitumor effect in hepatocellular carcinoma? Hepatol Res 2016;46:593-600. [Crossref] [PubMed]