Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases
Introduction
Hepatic stem/progenitor cells (HPCs) are stem cells residing in the most peripheral branches of the biliary tree (canals of Hering/bile ductules) (1); these cells are able to differentiate towards mature hepatocyte or mature cholangiocyte (2-4). In normal conditions, they are mostly quiescent cells taking no or minimal part in the renewal of liver parenchyma since the replacement of necrotic and apoptotic hepatocytes (or cholangiocytes) occurs through replication of adjacent hepatocytes within the lobules (or mature cholangiocytes within interlobular bile ducts) (4,5). However, this primary pathway is impaired easily by a variety of insults leading to the activation of a secondary proliferative pathway of HPCs. HPC activation/proliferation results in the appearance of reactive ductules (or ductular reaction: DR) which is composed by tortuous ductules, contain no discernable lumens (reactive ductules). Reactive ductules are anastomosing strands composed by progenitors with highly variable marker profiles maintaining cytokeratin (K) 7 and K19 in which intermediate cells of several differentiation states are continuously produced (4,5).
A HPC activation has been involved in the progression of chronic parenchymal diseases (chronic viral hepatitis) and chronic biliary diseases (such as Primary Biliary Cirrhosis: PBC) and in the occurrence of intrahepatic Cholangiocarcinoma (ICC) (6-10).
Several studies have suggested that DR drives hepatic fibrogenesis during liver injury (11,12). The resident stem/progenitor cell pool participates in the repair of liver damage either through the replacement of dead cells or by driving fundamental repair processes, including fibrosis and angiogenesis (13,14).
Vascular endothelial growth factor (VEGF) is a family of related growth factors that includes VEGF-A, -B, -C, -D, and -E and placenta growth factor (15). VEGF is a mitogen for vascular endothelial cells and regulates vascular pathophysiology, including vasodilatation, vascular permeability, migration, and survival of endothelial cells. The expression of VEGF and its receptors is not restricted to vascular endothelial cells; VEGF is secreted by several epithelia, where it modulates cell growth by autocrine and paracrine mechanisms. Three main receptors have been identified for VEGF (VEGFR), VEGFR-1 (Flt-1), VEGFR-2 (Flk-1), and VEGFR-3 (Flt-4). While VEGF-A interacts with VEGFR-1 and VEGFR-2, VEGF-C interacts with VEGFR-2 and VEGFR-3 (16-18).
A number of studies have shown that VEGF regulates the function of normal and hyperplastic cholangiocytes (16-18). Indeed, VEGF has been shown to stimulate cholangiocyte proliferation by an autocrine mechanism and to prevent cholangiocyte damage by interruption of apoptotic cascade (15). Moreover, VEGFs are potent inductors of vascularization, development and growth of several cancers, such as cholangiocarcinoma (19). Increased expression of VEGF plays an important role in regulating human cholangiocarcinoma growth. Overexpression of VEGF has been suggested to contribute to the ‘angiogenic switch’ of the malignant phenotype in human cholangiocarcinoma (19). Little information exists regarding the expression of VEGF by HPC in the course of liver non-malignant and malignant pathologies.
The aims of the present study are to evaluate the expressions of VEGFs and their receptors by HPCs and their relationships with the angiogenesis in chronic liver diseases.
Materials and methods
Materials
The study was carried out on liver biopsies taken for diagnostic purpose or on liver specimens obtained from explant livers; the materials were divided in the following groups:
- NL (N=5): liver biopsies with a normal histology from patients submitted to laparotomy;
- HCV-C (N=5): post-Hepatitic C Virus (HCV) liver cirrhosis;
- PBC (N=5): liver biopsies from postmenopausal female patients with diagnosis of PBC based on standard, internationally accepted criteria (Boyer 1997). PBC cases were classified as stage IV according to Ludwig criteria. All PBC patients and controls were negative for hepatitis B and C markers. All PBC patients were under treatment with ursodeoxycholic acid.
The diagnosis was based on histopathological examination of routinely processed tissue and on clinical and laboratory data. The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Light microscopy and immunohistochemistry (IHC)
Liver fragments (0.5 cm) were fixed in buffered formalin and embedded in low- paraffin, 3 µm sections were stained with haematoxylin-eosin and Masson’s trichrome. For immunohistochemistry (IHC) (20), sections were mounted on glass slides coated with 0.1% poly-L-lysine. After deparaffination, endogenous peroxidase activity was blocked by a 20-minute incubation in hydrogen peroxide (2.5%). Sections were incubated for one hour at room temperature with antibodies for (I) VEGF-A (Santa Cruz, Inc., Santa Cruz, CA; sc-152, rabbit polyclonal; 1:50); (II) VEGF-C (Santa Cruz, Inc., Santa Cruz, CA; sc-9047, rabbit polyclonal; 1:50); (III) VEGFR-1, VEGFR-2 and VEGFR-3 (Santa Cruz, Inc., Santa Cruz, CA; respectively sc-316 rabbit polyclonal; 1:50, sc-6261 rabbit polyclonal; 1:50 and sc-321 rabbit polyclonal; 1:50); (IV) anti-human Epithelial Cell Adhesion Molecule (EpCAM, Dako, mouse monoclonal; 1:50); (V) anti-human Von Willebrand Factor (vWF, Dako, mouse monoclonal; 1:50); and PCNA (Santa Cruz, Inc., Santa Cruz, CA; sc-7907, rabbit polyclonal; 1:50). Samples were then rinsed with PBS for 5 minutes, incubated for 20 minutes at room temperature with secondary biotinylated antibody (Dako LSAB Plus System, HRP, Milan, Italy), then with Dako ABC (Dako LSAB Plus System, HRP) and finally developed with diaminobenzidine (Dako). For immunofluorescence on cell culture, slides chambers were fixed in acetone for 10 min at room temperature and then rinsed with PBS-Tween 20. Non-specific protein binding was blocked by 5% normal goat serum. Fixed cells were incubated with primary antibodies. Then, cells were washed and incubated for 1h with labeled isotype-specific secondary antibodies (anti-mouse AlexaFluor-546, anti-mouse Alexafluor-488, anti-rabbit Alexafluor-488, anti-goat AlexaFluor-546, Invitrogen) and counterstained with 4,6-diamidino-2-phenylindole (DAPI) for visualization of cell nuclei. For all immunoreactions, negative controls (the primary antibody was replaced with pre-immune serum) were also included.
Sections were examined in a coded fashion by Leica Microsystems DM 4500 B Light and Fluorescence Microscopy (Weltzlar, Germany) equipped with a Jenoptik Prog Res C10 Plus Videocam (Jena, Germany). IHC observations were processed with an Image Analysis System (IAS-Delta Sistemi, Rome-Italy) and were independently performed by two researchers in a blind fashion. Semi-quantitative analysis of IHC positivity has been obtained counting in six non-overlapping fields (magnification ×20) for each slide. The evaluation of HPC compartment has been obtained measuring the area occupied by EpCAM positive cells within bile/reactive ductules as reported in previous paper (21). EpCAM positive HPCs alone or in small clamps localized in the parenchyma or at the portal interface have been added in these counts since they should be considered as a histological sectioning of bile/reactive ductules trough a transversal plane without any unique immunohistochemical markers, which distinguish them from cell within bile/reactive ductules. Typical cholangiocytes lining the interlobular bile ducts were not included in the counts. The area occupied by EpCAM+ HPCs was counted in the entire section and expressed as percentage in respect with the total parenchymal area (22).
The evaluation of angiogenesis has been obtained measuring the area occupied by vWF [a marker of blood vessel formation (23)] vessels and expressed as percentage in respect with the total parenchymal area.
Intermediate hepatocytes (IHs) were defined as cells with sizes between those of hepatocytes and HPCs (<40 but >6 µm in diameter), with faint CK-7 immunoreactivity in the cytoplasm and reinforcement at the plasma membrane. The presence of IHs was scored as reported elsewhere: 0= no IH, 1= single occasional IHs and 2= clusters of His (24).
The expression of VEGF-A, VEGF-C, VEGFR-1, VEGFR-2 and VEGFR-3, and PCNA by EpCAM-positive HPCs/DRs or by vWF-positive endothelial cells has been evaluated in serial sections and data were expressed as % of positive cells (25,26).
Statistical methods
Continuous normally distributed variables are summarized and represented graphically as mean ± standard error of means (SE). To compare the means between groups for normally distributed data, analysis of variance or the Student t test was performed. To determine differences between groups for not normally distributed data, medians were tested by Mann-Whitney U tests. The Pearson correlation coefficient was used to determine correlations between continuous normally distributed variables. The degree of association between nonparametric or ordinal variables was assessed using the Spearman nonparametric correlation. The association of independent factors with the degree of fibrosis was evaluated by stepwise multivariate logistic or linear regression analysis. Statistical significance was set to a P-value <0.05. Statistical analyses were performed by SPSS statistical software (SPSS Inc. Chicago IL, USA)
Results
HPCs in PBC and HCV-C biopsy samples
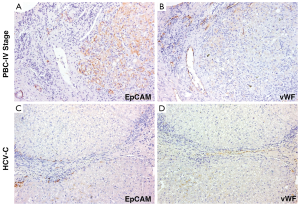
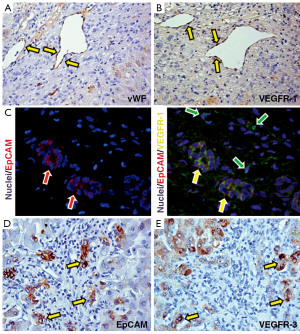
In pathological conditions (PBC and HCV-C), the HPCs activated and the reactive ductules compared. The extension of reactive ductules was higher in comparison with normal livers where normal bile ductules could be observed. PBC samples showed a more extensive expansion of HPC population (3.37±1.33 Epcam-positive cells) compared with those of HCV-C samples (0.78±0.46, P<0.01, Figure 1A,C). In cirrhotic samples, reactive ductules were located within the fibrous septa and at the interface with cirrhotic nodule (Figures 1,2); in PBC, several strands of progenitor cells penetrated deeply into cirrhotic nodules. Accordingly, the number of proliferating (PCNA+) ductular cells (PI-DR) was increased in pathological conditions (46.39±19.02) compared to normal controls (3.75±1.5, P<0.01); PI-DR was higher in PBC (62.45±9.22) in comparison with HCV-C (30.33±8.45, P<0.01, Figure 3).
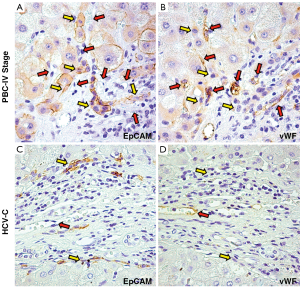
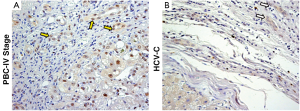
Interestingly, several EpCAM positive hepatocytes were present in strict relationship with reactive ductules. These Epcam positive cells could be considered as hepatocytes newly derived from HPCs, as showed elsewhere (27). Interestingly, the number of EpCAM positive hepatocytes was higher in PBC samples (1.75±0.5) compared with HCV-C (0.75±0.5, P<0.05).
As regard the extension of vascular structures (vWF positive vessels), a prominent increasing of vessels was observed in pathological specimens (Figure 1B,D) in comparison with normal livers. Moreover, PBC samples showed a more extensive angiogenesis (1.85±0.44 vWF-positive endothelial cells) if compared to normal livers and HCV-C biopsies (0.38±0.2, P<0.01).
In cirrhotic samples, vessels were mostly located within fibrous septa; interestingly, in PBC but no in HCV-C, vessels were present at the interface with cirrhotic nodule, were in strict relationship with reactive ductules and penetrated into nodules following HPCs and EpCAM positive Hepatocytes.
Overall, the extension of vascular structures was strictly correlated with the activation of HPCs (r=0.835, P<0.001)
Expression of VEGFs and VEGF-Rs in PBC and HCV-C biopsy samples
In normal liver, HPCs were almost negative for VEGFs (A and C), and VEGF-R (-R1, -R2, and -R3). In cirrhotic livers, VEGF-A and -C were expressed by hepatocytes in cirrhotic nodules and in reactive ductules. In particular, PBC samples (Figure 4) were characterized by an increased expression of VEGF-A (37.3±15.1) and VEGF-C (26.7±13.7) if compared to HCV-C samples (11.3±2.9, P<0.01 and 8.2±4.2, P<0.02, respectively) and the number of HPCs expressing VEGFs was correlated with the extension of ductular reaction and angiogenesis (r=0.813, P<0.001; r=0.749 P<0.01).
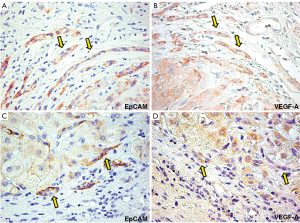
As regard VEGF-Rs, we found that VEGFR-1 and VEGFR-3 but not VEGFR-2 were expressed by HPCs and that their expression was higher in PBC samples (27.4±6.7 and 34.1±12.4) if compared to HCV-C (15.5±7.9 and 21.7±5.2, P<0.05). VEGFR-1 was also highly expressed by endothelial cells both in PBC and HCV-C (Figure 5).
Data are summarized in Table 1.

Full table
Discussion
Hepatic progenitor cells represent a reserve cell compartment that is activated only when the mature epithelial cells of the liver are continuously damaged or inhibited in their replication or in cases of severe cell loss (4,10). Numerous evidences indicated the activation of resident stem cell compartment in the majority of acute and chronic liver diseases. In chronic diseases, hHpSC highly proliferate and give rise to newly derived EpCAM positive hepatocyte in correlation with hepatocyte senescence (5,27). The activation of HPCs is characterized by the formation of so-called reactive ductules (2). A prominent ductular reaction has been previously described in chronic biliary diseases such as Primary Biliary Cirrhosis (PBC) and post-viral hepatitis C cirrhosis (28). However, less is known about the mechanisms of HPC niche activation and the remodelling of vascular bed. In this paper, we described that the niche activation is correlated with the appearance of neovessels. The biliary tree is nourished by terminal branches of the hepatic artery, which constitute a complex vascular system called Peribiliary Plexus (PBP). The peribiliary plexus, which extends up to the interlobular ducts flows into the hepatic sinusoids (29). Canals of Hering and part of bile ductules are not directly vascularized, and this could in part justify the quiescent state under normal conditions of the HPC compartment (29). Alterations of intrahepatic bile duct mass are associated with changes of the PBP architecture, thus supporting the increased nutritional and functional demands from the proliferated bile ducts (15). In previous works, we found a cross-talk mechanism between cholangiocytes and endothelial cells that mediates the adaptive changes of these cells during liver damage and involves the VEGF system.
In the present paper, we described a similar cross-talk between HPCs and endothelial cells during liver damaging; in fact, the extension of ductular reaction is associated with changes of the vascular architecture and with the increase of VEGF-A and VEGF-C protein expression in HPCs, thus supporting the increased nutritional and functional demands from the proliferated bile ducts.
The resident stem/progenitor cell pool participates in the repair of liver damage either through the replacement of dead cells or by driving fundamental repair processes, including fibrosis and angiogenesis (4,13). In this context, DR has been independently correlated with progressive fibrosis in adult and pediatric NASH and in HCV related cirrhosis (12,21,28). Here, we showed that different liver pathologies were able to elicit a different pattern of HPC niche activation. In particular, PBC is characterized by a more prominent ductular reaction in comparison with HCV-C; accordingly, the number of proliferating ductular cells was higher in comparison with HCV-C. In PBC samples, reactive ductules were located within the fibrous septa and at the interface with cirrhotic nodule and strands of progenitor cells penetrated deeply within cirrhotic nodules. In parallel, vessels were more numerous in PBC when compared with HCV-C, the vessels were in strict relationship with reactive ductules and followed HPCs penetrating into nodules. DR could be considered a main driver of fibrosis. DR might modulate hepatic fibrogenesis during liver injury through two possible mechanisms: (I) DR cells produce agents that are chemotactic for inflammatory cells and may activate HSCs (11), and (II) DR cells undergo epithelial-mesenchymal transition, contributing to the portal myofibroblast pool (14,30). Our results add a new aspect in this scenario suggesting that HPCs could trigger fibrogenesis by acting on endothelial cells within the niche and determining the proliferation of PBP with the appearance of newly-formed vessels within fibrous septa.
Our results have several implications in carcinogenesis. Recently, detailed studies on immunohistochemical profile has revealed that a whole range of phenotypical traits of hepatocytes, cholangiocytes and progenitor cells can be seen in liver primitive tumors (hepatocarcinoma and cholangiocarcinoma), being consistent with a origin from the hepatic stem cell compartment within canals of Hering (6,7,31). Since hepatic stem cell are activated in most chronic liver diseases that are known risk factors for the development of hepatocarcinoma (HCC) as well as cholangiocarcinoma (CCA), these cells are potential target cells for carcinogenesis (32).
Recently, a significant relationship has been identified between non-tumor periportal DR and overall survival in combined hepatocellular-cholangiocarcinoma (CHC) after curative treatment (22). CHC is a malignant primary liver tumor with poor prognosis that is thought to be of HPC origin. Background HPC activation is strongly associated with multifocal occurrence and related tumour recurrence, highlighting the critical role of background liver disease in the recurrence of CHC. Furthermore, the risk of intrahepatic tumor recurrence after resection strongly correlated with the proliferating (PI) DR (22). PI-DR appears to coincide with a field effect based on a noncancerous cellular responsiveness that predisposes to metachronous tumor recurrence in CHC suggesting that quantification of HPC activation may assist risk stratification for recurrence. That was supported by a correlation between PI-DR and coexistence of small cell change, which is thought to be a precancerous lesion of HCC (22). Libbrecht et al. described a close association between HPC marker expression and preneoplastic lesion of HCC (33). Although the evidence from humans is still fragmentary, HPC/DR is an attractive candidate as a tumorigenic target or stimulus. Thus, hepatic progenitor cells may be a double-edged sword promoting both liver regeneration and carcinogenesis in humans. Several papers underscored the important role of growth factors and VEGFs in the liver tumour (19,34,35); our results suggest that the capability of proliferating reactive ductules to produce vascular growth factors adds a new element in the mechanisms of malignant transformation which would be deeply investigate in future studies.
In conclusion, our results suggest an important role of VEGFs in support the expansion of HPC niche by an autocrine and paracrine effects on neighbouring cells stimulating the proliferation of HPCs and endothelial cells. These aspects could have important implication in fibrogenic processes and carcinogenesis.
Acknowledgements
E. Gaudio was supported by research project grant from the University “Sapienza” of Rome, FIRB grant # RBAP10Z7FS_001 and by PRIN grant # 2009X84L84_001. D. Alvaro was supported by FIRB grant # RBAP10Z7FS_004 and by PRIN grant # 2009X84L84_002.
Disclosure: The authors declare no conflict of interest.
References
- Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739-45.
- Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011;458:251-9.
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 2004;39:1477-87.
- Gaudio E, Carpino G, Cardinale V, et al. New insights into liver stem cells. Dig Liver Dis 2009;41:455-62.
- Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 2010;59:247-57.
- Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012;55:1876-88.
- Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008;47:1544-56.
- Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol 2003;163:1301-11.
- Theise ND, Kuwahara R. The tissue biology of ductular reactions in human chronic liver disease. Gastroenterology 2007;133:350-2.
- Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-45.
- Glaser SS, Gaudio E, Miller T, et al. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 2009;11:e7.
- Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 2007;133:80-90.
- Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132:415-31.
- Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 2008;118:3331-42.
- Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006;130:1270-82.
- Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol 2006;291:G307-17.
- Gaudio E, Franchitto A, Pannarale L, et al. Cholangiocytes and blood supply. World J Gastroenterol 2006;12:3546-52.
- Gaudio E, Onori P, Franchitto A, et al. Hepatic microcirculation and cholangiocyte physiopathology. Ital J Anat Embryol 2005;110:71-5.
- Fava G, Demorrow S, Gaudio E, et al. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int 2009;29:1031-42.
- Glaser S, Lam IP, Franchitto A, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010;52:204-14.
- Nobili V, Carpino G, Alisi A, et al. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 2012;56:2142-53.
- Cai X, Zhai J, Kaplan DE, et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology 2012;56:1804-16.
- Hicks C, Stevanato L, Stroemer RP, et al. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant 2012. [Epub ahead of print].
- Katoonizadeh A, Nevens F, Verslype C, et al. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int 2006;26:1225-33.
- Alvaro D, Onori P, Alpini G, et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol 2008;172:321-32.
- Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 2009;89:456-69.
- Yoon SM, Gerasimidou D, Kuwahara R, et al. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011;53:964-73.
- Clouston AD, Powell EE, Walsh MJ, et al. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 2005;41:809-18.
- Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology 1996;111:1118-24.
- Omenetti A, Choi S, Michelotti G, et al. Hedgehog signaling in the liver. J Hepatol 2011;54:366-73.
- Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138-51.
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68.
- Libbrecht L, Desmet V, Van Damme B, et al. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol 2000;33:76-84.
- Mancino A, Mancino MG, Glaser SS, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis 2009;41:156-63.
- Francis H, Onori P, Gaudio E, et al. H3 histamine receptor-mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res 2009;7:1704-13.


