A novel nomogram for predicting the prognosis of hepatocellular carcinoma patients following immune checkpoint inhibitors treatment beyond progression: a single center study based on Chinese population
Highlight box
Key findings
• This study is the first time to develop a column-line graphical model based on a comprehensive analysis of a variety of baseline clinical characteristics and laboratory test variables, which can accurately predict the prognosis of patients who continue to be immunized with checkpoint inhibitors after treatment with PD-1 monoclonal antibodies.
What is known and what is new?
• This study showed that immune drugs, mode of progression(lung), number of lines, Eastern Cooperative Oncology Group, and adverse effects (grade 3/4) were all prognostic factors of independent immunotherapy for liver cancer.
• This manuscript adds a validated prognostic nomogram for advanced hepatocellular carcinoma patients in the Chinese population, enhancing personalized treatment planning by accurately predicting survival outcomes post-immunotherapy beyond progression.
What is the implication, and what should change now?
• When immunotherapy is administered to patients with advanced liver cancer, it is necessary to further screen the advantageous beneficiaries for treatment, rather than for all liver cancer patients.
Introduction
Hepatocellular carcinoma (HCC) continues to be a significant global health concern, retaining its position as the sixth most prevalent cancer in 2020 and the third leading cause of cancer-related mortality (1,2). China, frequently designated as the ‘epicenter of HCC’, exhibits the highest global incidence and mortality rates for this disease. Each year, China witnesses approximately 466,000 new cases of HCC, positioning it at fourth place in terms of incidence, with an estimated 422,000 fatalities. The mortality rates attributed to HCC have now surpassed those of gastric cancer (GC), trailing solely behind lung cancer (3).
The treatment options for liver cancer are notably limited. Radical treatment and locoregional therapies are appropriate for HCC patients in the early to intermediate stages, while the majority of HCC patients receive systemic treatments (4). Despite the introduction of sorafenib, which has partially addressed the gap in first-line targeted therapy, its clinical effectiveness remains constrained. In Europe and the United States, patients have a median survival of 10.7 months, whereas Asian patients experience a significantly shorter median survival of only 6.5 months. The efficacy rate remains disappointingly low at a mere 3% (5,6).
The approval of subsequent targeted drugs, such as lenvatinib and regorafenib, has provided limited efficacy. The advancement of anticancer drugs is impeded by the presence of tumor heterogeneity, a challenge particularly emphasized in the context of liver cancer. The pronounced heterogeneity of liver cancer has hindered the identification of a specific “cancer gene dependency”, contributing to the constrained success of molecular targeted therapies. Nevertheless, the emergence of immune checkpoint inhibitors (ICIs) brings a ray of hope for patients who exhibit resistance or intolerance to targeted therapies.
PD-1, a type I transmembrane protein belonging to the immunoglobulin B7-CD28 family, consists of 268 amino acids and plays a suppressive role in human immune responses. Its main ligand, PD-L1, is primarily expressed on antigen-presenting cells and other non-hematopoietic cells. By engaging with PD-L1, PD-1 aids in activating the host immune system to combat cancer cells. ICIs targeting PD-1 and PD-L1 represent a prime example, functioning by blocking immune checkpoints to unleash the host immune system and enhance T cell-mediated attacks on tumor cells (7,8).
Immunotherapy has emerged as a significant breakthrough in the treatment of liver cancer. Currently, systemic therapy approaches for liver cancer include single-agent immunotherapy, combinations of immunotherapy with targeted therapy or chemotherapy, and dual immunotherapy. The phase III trial of IMbrave150 showed that atezolizumab (a PD-L1 inhibitor) combined with bevacizumab (an antiangiogenic agent) result in encouraging survival benefits compared to sorafenib alone (9,10).
Additionally, promising clinical trials such as ORIENT-32, HIMALAYA, and CARES-310 have firmly established the role of immunotherapy in the treatment of liver cancer (11-14).
However, the establishment of a standardized treatment protocol for disease progression following immunotherapy remains a challenge yet to be overcome.
ICIs distinguish themselves from conventional chemotherapy and targeted therapy by employing distinct mechanisms in combatting tumors. Consequently, the evaluation of tumor responses to immunotherapy differs from that of traditional treatments. Due to the potential for atypical delayed responses and pseudo-progression observed in patients undergoing ICIs, the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 is utilized for assessing progression-free survival (PFS) and objective response rate (ORR). However, this approach presents limitations when applied to immunotherapy evaluations, and alternative methods such as immune-related RECIST (irRECIST) and modified irRECIST have yet to gain widespread clinical acceptance (15,16).
In the field of immunotherapy, disease progression is commonly regarded as an indication of treatment failure, leading to the discontinuation of immunotherapy. However, certain studies propose the possibility of survival benefits associated with the continuation of PD-1 monotherapy following disease progression in patients with metastatic melanoma, lung cancer, and renal cell carcinoma (17-20). Nevertheless, the implications of maintaining PD-1 monotherapy after disease progression in HCC patients remain uncertain due to the insufficient availability of conclusive evidence.
To further investigate this matter, we employed nomograms, which are visual predictive tools grounded in relevant clinical and pathological parameters (21). Nomograms serve as widely utilized predictive models, providing estimations of the probability of clinical events.
To determine the potential benefits of continuing ICIs in advanced liver cancer patients following disease progression, we conducted a retrospective analysis of clinical data obtained from patients with late-stage liver cell carcinoma who received ICIs treatment. Our study aimed to assess the efficacy and safety of this treatment approach, and we developed a novel nomogram to predict the prognosis of liver cancer after ICIs therapy. We present this article in accordance with the TRIPOD reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-646/rc).
Methods
Patients
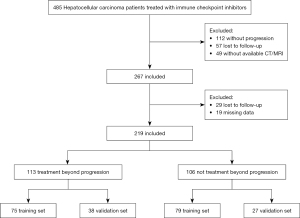
A retrospective analysis was conducted on a cohort of 485 patients with advanced HCC who received ICIs treatment at Nanjing Jinling Hospital between January 1, 2015, and August 31, 2022. Ultimately, a total of 219 patients met the inclusion criteria and were included in this study. The inclusion criteria encompassed the following: (I) age 18 years or older; (II) confirmed diagnosis of HCC through pathology or clinical diagnosis; (III) Barcelona Clinic Liver Cancer (BCLC) stage C or unsuitability for radical surgery or local treatment stage B; (IV) previous receipt of a minimum of two cycles of ICIs treatment and subsequent disease progression; (V) presence of at least one measurable lesion according to RECIST v1.1 criteria; and (VI) investigator’s assessment indicating potential clinical benefit from continuing ICIs treatment. Exclusion criteria consisted of: (I) development of intolerable toxicity following prior immunotherapy; (II) Eastern Cooperative Oncology Group (ECOG) score exceeding 3; and (III) fewer than two instances of PD-1 monotherapy administration following disease progression. The final follow-up date for this study was August 15, 2023. All participants provided informed consent, and the study adhered to the ethical principles outlined in the Declaration of Helsinki (as revised in 2013). Approval was granted by the Nanjing Jinling Hospital’s Ethics Committee (No. DZQH-KYLL-23-06).
Study design
Imaging evaluations were performed collaboratively by oncologists and radiologists. The assessment of treatment efficacy was based on the RECIST v1.1 criteria. The initial evaluation of treatment response occurred after two treatment cycles or as deemed necessary based on clinical considerations.
In this study, overall survival (OS) was defined as the duration from the initial progression following ICIs treatment to death from any cause. We examined various baseline characteristics, including age, gender, ECOG performance status, types of ICIs drugs, staging, baseline metastatic sites, site of progression, portal vein tumor thrombus (PVTT) grade, concurrent therapies, Child-Pugh stage, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), hemoglobin level, alpha-fetoprotein (AFP) levels (categorized into three groups: 1, <10 ng/mL; 2, 10–400 ng/mL; 3, >400 ng/mL), lactate dehydrogenase (LDH) level, white blood cell (WBC) count, and other relevant factors. Safety assessments were conducted for all eligible patients, and the severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
Patients were randomly divided into training and validation groups in a 7:3 ratio. Baseline characteristics of both patient groups were analyzed using chi-square or Fisher’s exact tests for categorical variables, with frequencies and proportions reported. Non-parametric tests were employed for continuous variables that did not follow a normal distribution, and the results were presented as the median and upper and lower quartiles.
Univariate Cox regression analysis was conducted in the training set to identify significant prognostic factors. Subsequently, significant variables were included in a multivariate Cox proportional hazards regression model to determine the association of each variable with the survival outcome of liver cancer patients, considering a significance level of P<0.05. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were used to express the results.
Independent influential factors were selected in the multivariate Cox regression model, and separate nomograms were constructed to predict the 6- and 9-month OS of liver cancer patients. The predictive accuracy of the nomograms was assessed using Harrell’s concordance index (C-index) and the area under the receiver operating characteristic (ROC) curve (AUC). Calibration plots were used to evaluate the accuracy of the nomograms in predicting the 6- and 9-month survival outcomes in liver cancer patients. Additionally, decision curve analysis (DCA) was performed to determine the clinical utility of the predictive models by quantifying the net benefits of decision-making guided by the nomograms. Methods for handling missing data include imputation and multiple imputation.
Furthermore, individual risk scores were calculated based on the developed nomograms, and two optimal cut-off points were determined. These cut-off points categorized patients into high-, intermediate-, and low-risk groups. Statistical significance was defined as a P value <0.05. All statistical analyses were performed using R version 4.2.1.
Results
Patient clinical characteristics
The study included a total of 219 patients, as depicted in the flowchart presented in Figure 1. These patients were randomly assigned to either the training set (n=154) or the validation set (n=65). Among the participants, 113 belonged to the treatment beyond progression (TBP) group, while 106 were classified in the not continuing TBP (NTBP) group. The distribution of PD-1 monoclonal antibodies was as follows: 119 patients received camrelizumab, 14 were administered sintilimab, 20 received toripalimab, 43 were treated with tislelizumab, and 23 were assigned other types of treatment (Table 1). The BCLC staging revealed that 41 patients were at stage B, while 178 were at stage C. In terms of age distribution, 15 patients were below 40 years, and 14 were above 70 years. The gender distribution consisted of 189 males and 30 females. Importantly, there were no significant statistical differences observed between the training and validation sets across various indicators, ensuring their comparability. Post-disease progression, the median OS (mOS) did not significantly differ between the TBP and NTBP groups (8.6 vs. 8.7 months, P=0.41) as depicted in Figure 2.
Table 1
| Variables | Total (n=219) | Training set (n=154) | Validation set (n=65) | P |
|---|---|---|---|---|
| TBP | 0.24 | |||
| No | 106 (48.40) | 79 (51.30) | 27 (41.54) | |
| Yes | 113 (51.60) | 75 (48.70) | 38 (58.46) | |
| Types of ICIs drugs | 0.96 | |||
| Camrelizumab | 119 (54.34) | 82 (53.25) | 37 (56.92) | |
| Nivolumab | 14 (6.39) | 11 (7.14) | 3 (4.62) | |
| Toripalimab | 20 (9.13) | 15 (9.74) | 5 (7.69) | |
| Sintilimab | 43 (19.63) | 30 (19.48) | 13 (20.00) | |
| Others | 23 (10.50) | 16 (10.39) | 7 (10.77) | |
| Staging | 0.61 | |||
| BCLC B | 41 (18.72) | 27 (17.53) | 14 (21.54) | |
| BCLC C | 178 (81.28) | 127 (82.47) | 51 (78.46) | |
| Liver metastasis | 0.30 | |||
| No | 46 (21.00) | 29 (18.83) | 17 (26.15) | |
| Yes | 173 (79.00) | 125 (81.17) | 48 (73.85) | |
| Lung metastasis | 0.60 | |||
| No | 158 (72.15) | 109 (70.78) | 49 (75.38) | |
| Yes | 61 (27.85) | 45 (29.22) | 16 (24.62) | |
| Lymph node metastasis | 0.39 | |||
| No | 158 (72.15) | 108 (70.13) | 50 (76.92) | |
| Yes | 61 (27.85) | 46 (29.87) | 15 (23.08) | |
| Bone metastasis | 0.72 | |||
| No | 191 (87.21) | 133 (86.36) | 58 (89.23) | |
| Yes | 28 (12.79) | 21 (13.64) | 7 (10.77) | |
| Other metastasis | 0.058 | |||
| No | 194 (88.58) | 141 (91.56) | 53 (81.54) | |
| Yes | 25 (11.42) | 13 (8.44) | 12 (18.46) | |
| PVTT grade | 0.60 | |||
| <3 | 134 (61.19) | 92 (59.74) | 42 (64.62) | |
| ≥3 | 85 (38.81) | 62 (40.26) | 23 (35.38) | |
| Probiotics | >0.99 | |||
| No | 198 (90.41) | 139 (90.26) | 59 (90.77) | |
| Yes | 21 (9.59) | 15 (9.74) | 6 (9.23) | |
| Age (years) | 0.72 | |||
| <40 | 15 (6.85) | 12 (7.79) | 3 (4.62) | |
| 40–70 | 190 (86.76) | 132 (85.71) | 58 (89.23) | |
| >70 | 14 (6.39) | 10 (6.49) | 4 (6.15) | |
| Gender | 0.30 | |||
| Male | 189 (86.30) | 130 (84.42) | 59 (90.77) | |
| Female | 30 (13.70) | 24 (15.58) | 6 (9.23) | |
| Surgery | 0.17 | |||
| No | 115 (52.51) | 86 (55.84) | 29 (44.62) | |
| Yes | 104 (47.49) | 68 (44.16) | 36 (55.38) | |
| Radiotherapy | 0.98 | |||
| No | 153 (69.86) | 107 (69.48) | 46 (70.77) | |
| Yes | 66 (30.14) | 47 (30.52) | 19 (29.23) | |
| TACE | 0.37 | |||
| No | 72 (32.88) | 54 (35.06) | 18 (27.69) | |
| Yes | 147 (67.12) | 100 (64.94) | 47 (72.31) | |
| HBV | >0.99 | |||
| No | 20 (9.13) | 14 (9.09) | 6 (9.23) | |
| Yes | 199 (90.87) | 140 (90.91) | 59 (90.77) | |
| HCV | >0.99 | |||
| No | 211 (96.35) | 148 (96.10) | 63 (96.92) | |
| Yes | 8 (3.65) | 6 (3.90) | 2 (3.08) | |
| Liver cirrhosis | 0.90 | |||
| No | 51 (23.29) | 35 (22.73) | 16 (24.62) | |
| Yes | 168 (76.71) | 119 (77.27) | 49 (75.38) | |
| Progression liver | 0.47 | |||
| No | 52 (23.74) | 34 (22.08) | 18 (27.69) | |
| Yes | 167 (76.26) | 120 (77.92) | 47 (72.31) | |
| Progression lung | >0.99 | |||
| No | 178 (81.28) | 125 (81.17) | 53 (81.54) | |
| Yes | 41 (18.72) | 29 (18.83) | 12 (18.46) | |
| Progression lymph node | 0.55 | |||
| No | 200 (91.32) | 139 (90.26) | 61 (93.85) | |
| Yes | 19 (8.68) | 15 (9.74) | 4 (6.15) | |
| Progression bone | 0.75 | |||
| No | 196 (89.50) | 139 (90.26) | 57 (87.69) | |
| Yes | 23 (10.50) | 15 (9.74) | 8 (12.31) | |
| Progression others | >0.99 | |||
| No | 195 (89.04) | 137 (88.96) | 58 (89.23) | |
| Yes | 24 (10.96) | 17 (11.04) | 7 (10.77) | |
| Line | 0.86 | |||
| 1 | 140 (63.93) | 97 (62.99) | 43 (66.15) | |
| 2 | 56 (25.57) | 41 (26.62) | 15 (23.08) | |
| 3+ | 23 (10.50) | 16 (10.39) | 7 (10.77) | |
| ECOG | 0.43 | |||
| 1 | 178 (81.28) | 122 (79.22) | 56 (86.15) | |
| 2 | 38 (17.35) | 30 (19.48) | 8 (12.31) | |
| 3 | 3 (1.37) | 2 (1.30) | 1 (1.54) | |
| AE grade 1/2 | 0.72 | |||
| No | 137 (62.56) | 98 (63.64) | 39 (60.00) | |
| Yes | 82 (37.44) | 56 (36.36) | 26 (40.00) | |
| AE grade 3/4 | 0.55 | |||
| No | 189 (86.30) | 131 (85.06) | 58 (89.23) | |
| Yes | 30 (13.70) | 23 (14.94) | 7 (10.77) | |
| Child-Pugh stage | 0.50 | |||
| A | 174 (79.45) | 120 (77.92) | 54 (83.08) | |
| B/C | 45 (20.55) | 34 (22.08) | 11 (16.92) | |
| AFP | 0.09 | |||
| 1 | 6 (2.74) | 6 (3.90) | 0 (0.00) | |
| 2 | 34 (15.53) | 20 (12.99) | 14 (21.54) | |
| 3 | 179 (81.74) | 128 (83.12) | 51 (78.46) | |
| NLR | 3.28 [2.43, 4.61] | 3.29 [2.37, 4.55] | 3.27 [2.64, 5.28] | 0.42 |
| Hemoglobin (g/L) | 130 [116.72, 136.24] | 130.55 [118, 136] | 128.59 [116, 138] | 0.96 |
| PLR | 136.53 [96.01, 168.84] | 136.48 [98.26, 168.23] | 137 [90.91, 178.57] | 0.82 |
| WBCs (×109/L) | 4.53 [3.8, 5.72] | 4.52 [3.8, 5.6] | 4.6 [4.2, 6.01] | 0.22 |
| Bilirubin (μmol/L) | 18.1 [14.57, 21.99] | 18.14 [14.64, 22.28] | 18.02 [13.7, 21.6] | 0.50 |
| Albumin (g/L) | 39.51 [36.7, 41.95] | 39.56 [36.8, 41.99] | 39.5 [36.44, 41.68] | 0.73 |
| LDH (U/L) | 219 [192, 265.48] | 222.5 [194.4, 271.72] | 215.08 [186, 245.78] | 0.18 |
Data are presented as n (%) or median [IQR]. AFP levels categorized into three groups: 1, <10 ng/mL; 2, 10–400 ng/mL; 3, >400 ng/mL. HCC, hepatocellular carcinoma; TBP, treatment beyond progression; ICIs, immune checkpoint inhibitors; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombosis; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HCV, hepatitis C virus; ECOG, Eastern Cooperative Oncology Group; AE, adverse event; AFP, alpha-fetoprotein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; WBC, white blood cell; LDH, lactate dehydrogenase; IQR, interquartile range.

Univariate and multivariate Cox regression analysis
Univariate Cox proportional hazards regression analysis was performed to identify potential prognostic factors influencing OS in liver cancer patients. The analysis revealed several factors that demonstrated significant associations with OS, including immunotherapies, lung metastasis, lung progression, line number, ECOG status, severe adverse events (grade 3/4), Child-Pugh score, NLR, PLR, and LDH. Furthermore, a multivariate Cox regression analysis was conducted to assess the independent risk factors for OS. The results indicated that immunotherapies, lung progression, ECOG status, and severe adverse events (grade 3/4) were identified as independent risk factors for OS, as shown in Table 2.
Table 2
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| TBP | |||||||
| No | Ref. | ||||||
| Yes | 1.412 | 0.939–2.122 | 0.10 | ||||
| Types of ICIs drugs | |||||||
| Camrelizumab | Ref. | Ref. | |||||
| Nivolumab | 4.018 | 1.906–8.47 | <0.001 | 4.955 | 2.278–10.779 | <0.001 | |
| Toripalimab | 1.739 | 0.843–3.589 | 0.13 | 1.49 | 0.702–3.161 | 0.30 | |
| Sintilimab | 2.612 | 1.48–4.609 | 0.001 | 2.596 | 1.429–4.716 | 0.002 | |
| Others | 0.752 | 0.368–1.535 | 0.43 | 0.903 | 0.427–1.908 | 0.79 | |
| Staging | |||||||
| BCLC B | Ref. | ||||||
| BCLC C | 2.130 | 1.135–3.997 | 0.020 | ||||
| Liver metastasis | |||||||
| No | Ref. | ||||||
| Yes | 0.869 | 0.514–1.469 | 0.60 | ||||
| Lung metastasis | |||||||
| No | Ref. | ||||||
| Yes | 1.523 | 0.986–2.353 | 0.058 | ||||
| Lymph node metastasis | |||||||
| No | Ref. | ||||||
| Yes | 1.044 | 0.677–1.608 | 0.85 | ||||
| Bone metastasis | |||||||
| No | Ref. | ||||||
| Yes | 1.603 | 0.932–2.758 | 0.09 | ||||
| Other metastasis | |||||||
| No | Ref. | ||||||
| Yes | 0.844 | 0.389–1.832 | 0.67 | ||||
| PVTT grade | |||||||
| <3 | Ref. | ||||||
| ≥3 | 1.114 | 0.74–1.675 | 0.61 | ||||
| Probiotics | |||||||
| No | Ref. | ||||||
| Yes | 0.947 | 0.504–1.776 | 0.86 | ||||
| Age (years) | |||||||
| <40 | Ref. | ||||||
| 40–70 | 0.575 | 0.296–1.115 | 0.10 | ||||
| >70 | 0.723 | 0.246–2.12 | 0.55 | ||||
| Gender | |||||||
| Male | Ref. | ||||||
| Female | 0.666 | 0.382–1.161 | 0.15 | ||||
| Surgery | |||||||
| No | Ref. | ||||||
| Yes | 0.971 | 0.646–1.46 | 0.89 | ||||
| Radiotherapy | |||||||
| No | Ref. | ||||||
| Yes | 1.164 | 0.754–1.797 | 0.50 | ||||
| TACE | |||||||
| No | Ref. | ||||||
| Yes | 1.257 | 0.818–1.932 | 0.30 | ||||
| HBV | |||||||
| No | Ref. | ||||||
| Yes | 2.288 | 0.997–5.251 | 0.051 | ||||
| HCV | |||||||
| No | Ref. | ||||||
| Yes | 0.743 | 0.234–2.355 | 0.61 | ||||
| Liver cirrhosis | |||||||
| No | Ref. | ||||||
| Yes | 1.568 | 0.946–2.601 | 0.08 | ||||
| Progression liver | |||||||
| No | Ref. | ||||||
| Yes | 0.626 | 0.394–0.996 | 0.048 | ||||
| Progression lung | |||||||
| No | Ref. | Ref. | |||||
| Yes | 1.678 | 1.035–2.722 | 0.04 | 1.997 | 1.206–3.306 | 0.007 | |
| Progression lymph node | |||||||
| No | Ref. | ||||||
| Yes | 1.277 | 0.696–2.345 | 0.43 | ||||
| Progression bone | |||||||
| No | Ref. | ||||||
| Yes | 1.224 | 0.69–2.172 | 0.49 | ||||
| Progression others | |||||||
| No | Ref. | ||||||
| Yes | 1.535 | 0.868–2.714 | 0.14 | ||||
| Line | |||||||
| 1 | Ref. | ||||||
| 2 | 1.270 | 0.808–1.997 | 0.30 | ||||
| 3+ | 1.965 | 0.992–3.891 | 0.053 | ||||
| ECOG | |||||||
| 1 | Ref. | Ref. | |||||
| 2 | 2.938 | 1.817–4.749 | <0.001 | 3.153 | 1.902–5.225 | <0.001 | |
| 3 | 4.401 | 0.596–32.493 | 0.15 | 11.959 | 1.471–97.246 | 0.02 | |
| AE grade 1/2 | |||||||
| No | Ref. | ||||||
| Yes | 1.418 | 0.933–2.155 | 0.10 | ||||
| AE grade 3/4 | |||||||
| No | Ref. | Ref. | |||||
| Yes | 1.774 | 1.059–2.971 | 0.03 | 2.512 | 1.45–4.353 | 0.001 | |
| Child-Pugh stage | |||||||
| A | Ref. | ||||||
| B/C | 1.401 | 0.897–2.189 | 0.14 | ||||
| AFP | |||||||
| 1 | Ref. | ||||||
| 2 | 4.023 | 0.901–17.961 | 0.07 | ||||
| 3 | 2.861 | 0.7–11.687 | 0.14 | ||||
| NLR | 1.022 | 0.94–1.112 | 0.61 | ||||
| Hemoglobin | 0.997 | 0.985–1.009 | 0.58 | ||||
| PLR | 1.000 | 0.998–1.003 | 0.74 | ||||
| WBCs | 0.959 | 0.863–1.066 | 0.44 | ||||
| Bilirubin | 1.013 | 0.986–1.04 | 0.35 | ||||
| Albumin | 1.002 | 0.997–1.007 | 0.49 | ||||
| LDH | 1.001 | 0.999–1.002 | 0.46 | ||||
AFP levels categorized into three groups: 1, <10 ng/mL; 2, 10–400 ng/mL; 3, >400 ng/mL. OS, overall survival; HR, hazard ratio; CI, confidence interval; TBP, treatment beyond progression; ref., reference; ICIs, immune checkpoint inhibitors; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombosis; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HCV, hepatitis C virus; ECOG, Eastern Cooperative Oncology Group; AE, adverse event; AFP, alpha-fetoprotein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; WBC, white blood cell; LDH, lactate dehydrogenase.
Nomograms for 6- and 9-month OS predictions
Nomograms were constructed to predict the 6- and 9-month OS based on the results obtained from the multivariate Cox regression model (Figure 3). The nomograms prominently feature immune drugs, lung progression, ECOG performance status, and severe adverse events as significant predictors for OS.

Nomogram validation
The calibration curves, presented in both the training and validation sets, demonstrated a high level of agreement between the predicted OS values generated by the nomograms and the observed values (Figure 4). In the training set, the C-index for OS was 0.732 (95% CI: 0.689–0.775), while in the validation set, it was 0.705 (95% CI: 0.616–0.795). Furthermore, the AUC values for the 6- and 9-month OS predictions in the training set were 0.863 (95% CI: 0.799–0.927) and 0.816 (95% CI: 0.735–0.897), respectively. In the validation set, the AUC values for the same time points were 0.891 (95% CI: 0.795–0.986) and 0.788 (95% CI: 0.666–0.911) (Figure 5). These findings emphasize the strong discriminative capacity of the nomograms.


Clinical application of the nomograms
The application of DCA to assess the clinical utility of the nomograms for OS yielded promising results in both the training and validation sets (Figure 6). Utilizing the derived risk scores and optimal cutoff values from the nomograms, patients were effectively stratified into high-risk (score >47.59), intermediate-risk (score between 37.50 and 47.59), and low-risk groups (score <37.50). Kaplan-Meier survival curves demonstrated significant distinctions among these risk groups, further validating the predictive capacity of the nomograms (Figure 7).


Discussion
HCC is a prominent contributor to cancer-related mortality worldwide. In fact, the mortality rate associated with HCC is escalating at a more rapid pace compared to other types of cancer (22). This alarming rise in HCC-related mortality emphasizes the increasing significance of this disease.
Immunotherapy, particularly the utilization of ICIs to activate T lymphocytes and induce anti-tumor immune responses, has emerged as a fundamental treatment modality in clinical practice (23). In the management of liver cancer, immunotherapy has become a primary therapeutic strategy. In liver cancer management, it now serves as a primary therapeutic approach. Concurrently, the combination of transarterial chemoembolization (TACE) with systemic therapy offers a promising treatment alternative for patients with advanced liver cancer (4). Additionally, in the realm of liver cancer adjuvant therapy, numerous clinical trials are underway, showing significant potential (24).
Immunotherapy for liver cancer does not have definitive predictive markers; however, some studies suggest that certain indicators may predict the efficacy of immunotherapy in liver cancer. Gender is also one of the factors affecting the efficacy of immunotherapy, with similar benefits among men and women in immune combination therapy, but more benefits among men in the population treated with immune drug monotherapy (25). Peripheral blood biomarkers can predict the prognosis of HCC patients treated with PD-1 antibodies. The development of nomogram models can help screen potential patients who may benefit from immunotherapy. However, the sample size included in this study was limited, and further expansion of the sample is needed to confirm the conclusions of this study (26). Meanwhile, AFP can also be used as a predictor of the efficacy of immunotherapy in liver cancer (27).
A novel approach known as cross-line therapy has gained attention, involving the modification of drug regimens post-progression in the first-line treatment while retaining potentially beneficial drugs. This approach often incorporates agents with cytotoxic properties that can enhance tumor vasculature or modulate the immune microenvironment. The efficacy of cross-line therapy has been explored in various cancer types. For instance, in breast cancer, it combines anti-HER2 monoclonal antibodies with chemotherapy to achieve synergistic effects (28). In colon cancer and non-small cell lung cancer (NSCLC), the integration of anti-angiogenesis treatment with chemotherapy has shown promising results (29). Currently, we are pioneering a study focusing on PD-1 inhibitor cross-line therapy for liver cancer.
A comprehensive study involving 799 patients with solid tumors participating in various clinical trials investigated the continuation of pembrolizumab treatment (referred to as TBP) after disease progression. This cohort consisted of patients with melanoma, NSCLC, GC, head and neck squamous cell carcinoma (HNSCC), clear cell renal cell carcinoma (ccRCC), and urothelial carcinoma (UC). The study findings demonstrated that following disease progression, a notable percentage of patients (ranging from 8.9% to 24.4%) experienced a minimum 30% reduction in lesion size, while a substantial proportion of patients (ranging from 64.8% to 75.9%) maintained stability in the size of target lesions during advanced disease stages. These observations suggest potential clinical benefits associated with the continuation of ICI treatment after disease progression (30). The increasing adoption of TBP treatment can be attributed to improved understanding of the efficacy and safety profiles of PD-1 inhibitors, as well as the decreasing costs of these drugs. Previous study has demonstrated that comprehensive analysis using nomograms can accurately predict clinical outcomes for metastatic melanoma patients undergoing ICI therapy (31). However, the effectiveness and prognostic stratification of persistent PD-1 monotherapy after disease progression in advanced liver cancer patients treated with PD-1 antibodies remain significant challenges in the Chinese population.
Through Cox multivariate analysis, we identified several independent prognostic factors, including the use of immunotherapies, site of lung progression, treatment lines, ECOG performance status, and occurrence of grade 3/4 adverse reactions. Patients who received pembrolizumab, experienced non-lung disease progression, had favorable ECOG scores, and did not have a history of grade 3/4 immune-related adverse events may derive enhanced benefits from “TBP”. To facilitate risk stratification, we developed a nomogram utilizing these four independent risk factors. The ROC curve analysis effectively categorized patients into high-, medium-, and low-risk groups. Importantly, the low-risk group exhibited significantly improved OS compared to the high-risk group. The calibration curve demonstrated a close alignment between the nomogram’s predicted probabilities and the actual outcomes. Our predictive model for OS in liver cancer patients achieved a C-index exceeding 0.7 in both the training and validation sets, indicating its robust predictive accuracy. Furthermore, the calibration plot confirmed that the nomogram accurately predicted the 6- and 9-month OS outcomes. DCA highlighted the superior clinical utility of the nomogram-based prognostic model, which was further validated by the AUC (32). The DCA emphasized the practical value of the prognostic model based on the nomogram, supported by the AUC.
It is important to note that this study represents a retrospective real-world analysis, which provides a more authentic clinical context compared to conventional clinical trials. Real-world studies, characterized by broader inclusion criteria, effectively capture the diversity of patients, offering a more comprehensive assessment of treatment efficacy and safety across a wider population. However, it is crucial to acknowledge that such studies are prone to biases and confounding factors, underscoring the need for larger sample sizes to mitigate potential limitations and strengthen the study’s conclusions (33).
One limitation of our study is its retrospective design conducted at a single center, which resulted in a relatively small sample size and included non-randomized patients, potentially introducing selection bias. Although our internal validation demonstrated the robustness and applicability of the prognostic model, external validation is essential, particularly in populations with distinct etiology and incidence rates that differ significantly from the Chinese demographic. To validate and further explore the findings of our study, future efforts should encompass extensive multinational research and multicenter clinical trials. These endeavors will contribute to the authentication and broader generalizability of our prognostic model.
Conclusions
This study meticulously analyzed the impact of continuing immunotherapy beyond disease progression in HCC patients, employing a robust dataset of 219 individuals. Through comprehensive univariate and multivariate Cox regression analyses, it identified key prognostic factors influencing OS, including the type of immunotherapy, lung progression, ECOG performance status, and the occurrence of severe adverse events. Notably, the study developed and validated predictive nomograms for 6- and 9-month OS, incorporating these significant predictors. The calibration curves and C-index values underscored the nomograms’ accuracy in both the training and validation sets, demonstrating their strong discriminative capacity. Furthermore, DCA confirmed the clinical utility of these nomograms, enabling effective stratification of patients into distinct risk groups with significant survival differences. This research highlights the potential benefits of tailored treatment strategies based on individual risk assessments in HCC management, advocating for the nuanced application of immunotherapy beyond conventional treatment thresholds.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-646/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-646/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-646/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-646/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study adhered to the ethical principles outlined in the Declaration of Helsinki (as revised in 2013) and received approval from the Nanjing Jinling Hospital’s Ethics Committee (No. DZQH-KYLL-23-06). All participants provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598-606. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Brandi G. Trans-Arterial Chemoembolization Plus Systemic Treatments for Hepatocellular Carcinoma: An Update. J Pers Med 2022;12:1788. [Crossref] [PubMed]
- Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 2018;29:1402-8. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Berger KN, Pu JJ. PD-1 pathway and its clinical application: A 20year journey after discovery of the complete human PD-1 gene. Gene 2018;638:20-5. [Crossref] [PubMed]
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72:917-27. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Brandi G. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy 2021;13:637-44. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. [Crossref] [PubMed]
- Peng Y, Zeng X, Peng L, et al. Sintilimab Plus Bevacizumab Biosimilar Versus Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Front Pharmacol 2022;13:778505. [Crossref] [PubMed]
- Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet 2023;402:1133-46. [Crossref] [PubMed]
- Kelley RK, Sangro B, Harris W, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol 2021;39:2991-3001. [Crossref] [PubMed]
- Blumenthal GM, Theoret MR, Pazdur R. Treatment Beyond Progression With Immune Checkpoint Inhibitors-Known Unknowns. JAMA Oncol 2017;3:1473-4. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ishihara H, Takagi T, Kondo T, et al. Nivolumab treatment beyond progression for metastatic renal cell carcinoma: New role of metastasectomy in the immune checkpoint inhibitor era? Int J Urol 2020;27:691-2. [Crossref] [PubMed]
- Boku N, Satoh T, Ryu MH, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 2021;24:946-58. [Crossref] [PubMed]
- Won SE, Park HJ, Byun S, et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Oncoimmunology 2020;9:1776058. [Crossref] [PubMed]
- Chen C, Xiong X, Cheng Y, et al. Expanding the applications of immune checkpoint inhibitors in advanced lung cancer beyond disease progression. Front Immunol 2023;14:1266992. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312-37. [Crossref] [PubMed]
- Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2872-8. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol 2020;16:2587-9. [Crossref] [PubMed]
- Santoni M, Rizzo A, Mollica V, et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol 2022;170:103596. [Crossref] [PubMed]
- Jia G, Qiu L, Zheng H, et al. Nomogram for predicting survival in patients with advanced hepatocellular carcinoma treated with PD-1 inhibitors: incorporating pre-treatment and post-treatment clinical parameters. BMC Cancer 2023;23:556. [Crossref] [PubMed]
- Zhang Y, Shen H, Zheng R, et al. Development and Assessment of Nomogram Based on AFP Response for Patients with Unresectable Hepatocellular Carcinoma Treated with Immune Checkpoint Inhibitors. Cancers (Basel) 2023;15:5131. [Crossref] [PubMed]
- Hammerman A, Greenberg-Dotan S, Feldhamer I, et al. Second-Line Treatment of Her2-Positive Metastatic Breast Cancer: Trastuzumab beyond Progression or Lapatinib? A Population Based Cohort Study. PLoS One 2015;10:e0138229. [Crossref] [PubMed]
- Takeda M, Yamanaka T, Seto T, et al. Bevacizumab beyond disease progression after first-line treatment with bevacizumab plus chemotherapy in advanced nonsquamous non-small cell lung cancer (West Japan Oncology Group 5910L): An open-label, randomized, phase 2 trial. Cancer 2016;122:1050-9. [Crossref] [PubMed]
- Topp BG, Channavazzala M, Mayawala K, et al. Tumor dynamics in patients with solid tumors treated with pembrolizumab beyond disease progression. Cancer Cell 2023;41:1680-1688.e2. [Crossref] [PubMed]
- Pires da Silva I, Ahmed T, McQuade JL, et al. Clinical Models to Define Response and Survival With Anti-PD-1 Antibodies Alone or Combined With Ipilimumab in Metastatic Melanoma. J Clin Oncol 2022;40:1068-80. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Elkin EB, et al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 2008;8:53. [Crossref] [PubMed]
- Wang W, Tan J, Ren Y, et al. Real-world data studies: update and future development. Chinese Journal of Evidence-Based Medicine 2020;20:1241-6.