Nutrition of the critically ill - emphasis on liver and pancreas
Health care-associated infections - a common cause of death
Advanced surgical and medical treatments, as well as medical and surgical emergencies are, despite some breath-taking advances in medico-pharmaceutical and surgical treatment still affected by an unacceptably high morbidity and mortality The incidence of health care-associated infections (HCAI), to the largest extent consisting in sepsis, has in most recent years increased dramatically, much in parallel to and associated with increased use of invasive technologies, and is suggested today to constitute the fourth leading cause of disease in industrialized countries (1). In fact HCAI constitutes one of the fastest, if not the fastest, growing and unsolved problems in modern medicine. With the present rate of increase, it has the potential to at least double to the year 2050. As a matter of fact, sepsis is estimated to affect at least 18 million individuals worldwide, and with mortality rates of 25% to 30% (2,3), severe sepsis kills more individuals annually than prostate cancer, breast cancer, and HIV/AIDS combined, and the numbers of cases are increasing every year (4). Yet it is a condition that is poorly understood by those outside of medicine.
More than 230 million major surgical procedures are estimated to be undertaken each year worldwide (5). It has been calculated that around 25 million patients will each year worldwide undergo high-risk surgery, and no less than 3 million will not make it to be discharged from hospital (6). A recent study followed 46,539 adult patients undergoing standard inpatient non-cardiac surgery at 498 hospitals across 28 European nations. Four percent of included patients died before discharge, a significantly higher mortality rate than expected (6), the lowest observed in Estonia, Finland, Iceland, Norway, Netherlands and Sweden, and the highest registered in Belgium, Croatia, Ireland, Italy Latvia, Poland, Romania and Slovakia, findings strongly associated with access to critical care. As a matter of fact, those, who died (73%) were not admitted to critical care at any stage; most patients who died (73%) were not admitted to critical care at any stage after surgery. Furthermore, of patients who died after admission to critical care, did almost half (43%) die after being returned to the standard ward (6).
Complication after surgical procedures remains an important cause of death (7-10), and has also in fact increased during the last decades. Furthermore, patients who develop complications and might survive and leave hospital are known to continue to suffer reduced functional independence and also suffer reduced long-term survival (7,11-13). About 10% of the patients who today undergo surgery are known to develop complications and about 80% of all postoperative deaths are as a fact registered (8-10) as infections. It is especially important that the characteristics of these patients as well as the risk of various treatments are identified.
Artificial nutrition - a major contributor to sepsis
We are increasingly aware of the negative influence of obesity and various chronic diseases on outcome, but also the negative influence of the highly artificial environment in modern ICUs, as well as the risks associated with use of pharmaceuticals, artificial nutrition, assisted ventilation, use of skin penetration devises etc on outcome, even if we not always seem to consider them in daily practice as we should. A recent multivariate analysis identified emergency surgery, mechanical ventilation, fluid resuscitation, and use of vasoactive drugs in the postoperative period as the strongest indicators of risk of sepsis (14). Other studies suggest use of artificial feeding regimens, both enteral and parenteral, as a major contributor to ICU-associated sepsis; catheter-related sepsis is reported to occur in about 25% of patients fed via intravenous feeding-tubes (15). Other common perioperative practices, e.g., preoperative antibiotics (16), and mechanical bowel preparation (17,18) will as a matter of fact, instead of preventing expected infections, contribute to increased rates of treatment-associated infections. Other measures in the ICU such as mechanical ventilation (19) use of various pharmaceutical drugs, antibiotics (20,21) as well as hemo-therapeutics, chemical solutions for clinical nutrition and a number of other pharmaceuticals promote super-inflammation and, indirectly, infection.
The trauma/operation-induced acute phase response and increased inflammatory reaction in the body is often aggravated by supply of pharmaceuticals of diverse nature, which contribute to paralyzing immune functions, and impairment of neutrophil and macrophage functions. The inflammation is made worse by aggressive supply of nutrients, especially with the use of the parenteral route, but also seen when the enteral route is applied. Herndon et al. demonstrated already in 1989 in their now classical study undertaken in burn patients a great advantage of feeding the enteral (EN) route instead parenteral (PN). Identical solutions were either supplemented as PN or EN, a dramatic decrease in mortality rate observed; 26% after EN compared to 63% after PN (P<0.05). Hyperalimentation should no longer be routine practice during the first 10-14 days. Administration of larger amounts of fluid and electrolytes (22-24), fat (25-27), sugars (28-30), macromolecules/colloids applied intravascularly such as dextrans (31) and hydroethylstarch (HES) increases immune dysfunction, impairs resistance to disease and increases morbidity.
Colloids contribute to increased rate of infections
We demonstrated already 35 years ago in an experimental study comparing the effects of 0.9% NaCl solution, dextran 70, hydroxyethyl starch, degraded gelatin and fat emulsion that dextran 70, hydroxyethyl starch and degraded gelatin caused both significant hemodilution and decreased platelet count (32). Our subsequent studies demonstrated that dextrans, degraded gelatin and hydroxyethyl starch, all cause increased APT time, impaired ADP- and collagen-induced aggregation and induce a significantly increased bleeding time and blood loss after experimental liver resection (33), Another study of ours to the effects of dextran in experimental pneumococcal infections demonstrated a significantly increased mortality (59%) as well as a significantly increased number of abscesses when compared to the mortality (23)% in animals treated with only saline (P<0.05) (34). When the susceptibility to induced pneumococcal peritonitis was studied after supply of dextrans or fat emulsions (Intralipid); both inducing significantly higher mortality rates; dextran 80% (P<0.01), fat emulsions 47% (P>0.05) compared to saline (20%) (35).
It is not easy to understand that both HES and dextrans continue to be used despite the fact that a Cochrane study already in the year 2000 found no advantages of them over crystalloids (36). A recent report under the auspices of European Society of Intensive Care Medicine (ESICM) analyzed the experience of colloid treatment in mixed intensive care units (ICU), especially in cardiac surgery, head injuries, sepsis and organ donor patients, as reported in various meta-analyses, systematic reviews and clinical studies, concluding “We recommend not to use colloids in patients with head injury and not to administer gelatins and HES in organ donors. We suggest not using hyperoncotic solutions for fluid resuscitation. We conclude and recommend that any new colloid should be introduced into clinical practice only after its patient-important safety parameters are established” (37). It should especially avoided in liver surgery particularly in liver resections with its dramatically reduction in immune cells and parenchymal cells. 798 patients with severe sepsis were in a study performed by the Scandinavian Critical Care Trials Group randomly assigned in the ICU to fluid resuscitation with either 6% HES 130/0.42 (Tetraspan®, Braun Medical Supplies) or Ringer’s acetate (Sterofundin ISO®, BraunMedical Supplies); the HES-supplied patients reported to suffer an increased risk of death within 3 months and more likely to require renal-replacement therapy, as compared with those receiving Ringer’s acetate (38).
Crystalloids often enough during the first two weeks
In fact and as observed already about 15 years ago that large amounts of calories seem not to be needed during the first 10-14 days after common surgery or trauma, as demonstrated in two well-designed randomized studies involving 300 and 195 resp. patients, who underwent major general surgery in Scandinavia (39), and in the US (40) resp. In the first study, which lasted for up to 15 days, supply of total parenteral nutrition (TPN) was compared to infusion of only 1,000-1,500 kcal/day of glucose. The nitrogen loss during the first week was reduced to about half in the glucose-supplied group compared to the TPN group, but no differences in morbidity or mortality were observed, and the authors concluded “overfeeding seems to be a larger problem than underfeeding” (39). In the second study, performed at Memorial-Sloan-Kettering Cancer Center in New York, the patients were randomized allocated to either early enteral supplementation with a so called immuno-enhancing diet (Impact®, Novartis) or only iv crystalloid infusions. The daily intake of calories was low in both groups, 1,000 and 400 kcal that is 61% and 22% of defined goals (25 kcal/kg/day). No differences in number of minor, major or infectious complications, number of wound infections, mortality or length of stay were observed between the groups (40).
Numerous experimental and clinical studies have demonstrated that also enteral nutrition formulas commonly are deleterious to the immune functions and, as often observed especially will reduce microbiota and impair gut barrier functions. It was demonstrated in experimental animals already 20 years ago that various commercial clinical nutrition formulas will almost immediately induce loss of intestinal barrier function, promote bacterial translocation, and impair host immune defense (41), a phenomenon, today often observed in humans. The experimental studies demonstrated a dramatic increase in incidence of bacterial translocation to the mesenteric lymph node when the animals were fed nutrition formulas such as Vivonex® (53%), Criticare® (67%), or Ensure® (60%) (P<0.05) (20,41,42). Significant elevations in pro-inflammatory cytokines have also been observed in patients after pancreat-duodenectomy and fed a standard enteral nutrition solution (Nutrison®); IL-1beta day 7 (P<0.001); day 14 (P=0.022), TNF-alpha- day 3 (P=0.006); day 7 (P<0.001). It is of special interest that such changes were no longer seen when the standard nutrition was replaced by a formula, claimed to have immune-modulatory effects (Stresson®); instead anti-inflammatory cytokines were observed to be significantly elevated: IL-1ra/s: day 7 (P<0.001); IL-6: day 10 (P=0.017); IL-8: day 1 (P=0.011) days 3, 7, 10, and 14 (P<0.001), and IL-10: days 3 &10 (P<0.001) (43).
Immuno-preventive nutrition in sight?
The tolerance to LPS of commercial diets was studied in exp. animals divided into the following three groups of diet: (I) control diet receiving standard soy-based diet rich in cysteine, crude fibers and ω-6 PUFA linoleic acid but devoid of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), (II) a 100% whey-peptide-based commercially available liquid diet (Peptaman AF, Nestlé) high in cysteine, as well as in EPA, DHA and prebiotic fructooligosaccharides (FOS) and (III) a casein-based liquid isonitrogenous diet (Promote® Very High Protein Nutrition, Abbott), low in cysteine and devoid in EPA, DHA and FOS. The whey-peptide-based diet rich in EPA-DHA, cysteine, and FOS protected the animals significantly better than the two other diets against specific and general effects of systemic inflammatory syndrome but also against damage to tissues particularly the liver (44).
The effect of early enteral nutrition with a new immune-modulatory diet (IMD) enriched with hydrolyzed whey peptide (HWP), a protein complex derived from milk, and suggested to have anti-inflammatory effects was recently studied (MHN-02, MEIN®, Meiji Dairies Co., Tokyo, Japan) in 40 patients after living-donor liver transplantation (LDLT). The treated patients demonstrated when compared to 36 patients, who received a conventional elemental diet (Elental®, Ajinomoto Pharma Co, Tokyo, Japan (control group) a considerably reduced incidence in bacteremia in the HWP group (15%) than in the control group (47%) (P=0.002). Although it did not reach statistical significance a reduced incidence of infection-induced mortality was also observed (P=0.145) (45).
Over-reacting neutrophils
Dysbiosis-associated systemic inflammation is almost regularly observed in severe trauma and after surgery, accompanied by severe leakage of endotoxin into the body, and leading to infection and sometimes severe sepsis. As a consequence a significant decrease in lymphocytes, and a significant, often disproportionate, increase in both circulating and tissue neutrophils, paralleled by a persistent decline in T-4 helper lymphocytes and elevation of T-8 suppressor lymphocytes will occur (46). It is suggested that a T-4/T-8 lymphocyte cell ratio of <1 is a sign of severe immunosuppression and prediction of poor outcome in conditions such as multiple and severe trauma, multiple organ dysfunction syndrome, severe acute pancreatitis and in in myocardial infarction and stroke, as well as during chemotherapeutic treatments, particularly in oncology patients (47).
A large early increase in circulating neutrophils is always accompanied by tissue infiltration of neutrophils, responsible for common post-trauma/postoperative dysfunctions such as paralytic ileus (48,49), bone marrow suppression, endothelial cell dysfunction, and responsible for tissue destruction and organ failure, particularly in the lungs (50-53), intestines (50), liver (54) and kidney (55). Neutrophil infiltration to distant organs (56), particularly the lungs (52), is significantly aggravated by mechanical therapeutic efforts such as handling of the bowels during operation (48), and ventilation of the lungs (57). Poor nutritional status, preexisting immune deficiency, obesity, diabetes and in high levels of blood sugar (58) contribute to immune deterioration and to increased expressions of molecules such as NF-κB, COX-2, LOX and iNOS (58,59).
It is important to remember that a disproportionate increase in circulating neutrophils can, to a large extent, be successfully inhibited by the supplementation of antioxidants (60-62) as well as specific probiotics [258]. Supplementation of probiotics is also shown to effectively prevent neutrophil infiltration of the lung and reduce subsequent tissue destruction, as demonstrated in studies with inflammation induced by cecal ligation and puncture (CLP) - see further below.
Bioecological reduction of inflammation, neutrophil infiltration and tissue destruction
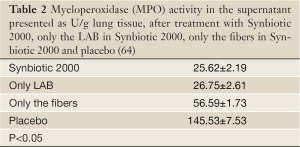
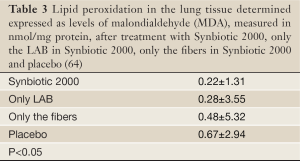
Experimental animals, subjected to induced infections through ceacal ligation and puncture (CLP), were treated with prophylactic supplementation using a synbiotic cocktail, Synbiotic 2000 Forte (see further below). The treatment consisted in a cocktail of the four LAB, which either was injected subcutaneously at the time of trauma (63) or supplied orally together with prebiotic fibers as a pretreatment for three days before induction of trauma (64). Both treatments effectively prevented neutrophil accumulation in the lung tissues (Table 1) as well as pulmonary tissue destruction (Figure 1). Significant reductions in parameters associated with the degree of systemic inflammation, such as myeloperoxidase (MPO, Table 2), malondialdehyde (MDA, Table 3) and nitric oxide (NO, Table 4), indicated a significant suppression of trauma-induced inflammation, all differences between the treatment and placebo groups in the two studies being statistically significant (<0.05) (64).

Full Table
Full Table
Full Table

Full Table
Life-threatening systemic inflammation
A study of patients in intensive care suffering life-threatening extreme systemic inflammation, what is called “systemic inflammation response syndrome” (SIRS) - and its relation to gut microbiota was recently published. Gut microbiota in twenty-five patients with severe SIRS and a level in serum of C-reactive protein above 10 mg/dL were analysed (65). and markedly lower total anaerobic bacterial counts, particularly of the beneficial Bifidobacterium and Lactobacillus observed, paralleled by higher counts of total facultative anaerobes such as Staphylococcus and Pseudomonas compared to healthy volunteers, Gram-negative facultative anaerobes were the most commonly identified microbial organisms in mesenteric lymph nodes and at serosal scrapings at laparotomy. Gastrointestinal complications were strongly associated with a significantly reduced number of total obligate anaerobes and highly increased numbers of Staphylococcus and Enterococcus and significantly decreased numbers of total obligate anaerobes and total facultative anaerobes (65).
A more recent study in 63 similar patients suggests impaired gastrointestinal motility as a significant marker of poor outcome (66). Patients with ≥300 mL per day reflux from nasal gastric feeding tube demonstrated significantly lower numbers of total obligate anaerobes including Bacteroidaceae and Bifidobacterium, higher numbers of Staphylococcus, lower concentrations of acetic acid and propionic acid, and higher concentrations of succinic acid and lactic acid (P≤0.05), accompanied by dramatically higher incidences of bacteremia (86% vs. 18%) and mortality (64% vs. 20%) than patients without gastric detention (P≤0.05) (66). Furthermore, in 29 similar patients treatment with a synbiotic composition, consisting of Bifidobacterium breve and Lactobacillus casei, in combination with galactooligosaccharides, was attempted. Higher levels of Bifidobacteria and Lactobacillus, but also total organic acids, particularly short-chain fatty acids, were reported and the incidence, compared to historical controls, of infectious complications such as enteritis, pneumonia, and bacteremia, observed to be significantly lower in the treated group (67).
Personal experience with pro- and synbiotics
My personal interest in microbiota and probiotics started in the early 1980’s. Since 1963 I have been involved in the development of extensive liver surgery and active in the search for new tools to combat the unacceptably high rate of peri-operative infections, which was and still is associated with major surgery in general and in particularly with extensive liver resections. At that time it was standard practice to treat patients with an antibiotic umbrella for at least the first five post-operative days, in the belief that this treatment would reduce the rate of post-operative infections. However, a review of our last 81 liver resections gave unexpected information, which directed my interest to human microbiota and the possibility of using probiotics as an alternative infection prophylaxis. From this study it was shown that only 57/81 patients had, in fact, received antibiotic treatment; this prophylaxis had been neglected in the remaining 24/81 patients (68,69). It was surprising that there were no cases of sepsis in the group of patients, who had not received prophylactic antibiotics with sepsis incidence. There was at that time a growing awareness of the importance of human microbiota (70) and to contemporaneously published studies that had attempted to recondition the gut through supply of lactobacilli (71).
There was also at that time a growing understanding that not only disease but lifestyle and chemicals and pharmaceuticals, could impair microbiota and immune defense. The use of probiotic treatment, as alternative means of preventing unwanted infections in disease in general but particularly in surgical and medical critically ill patients, appeared an attractive option. This was the reason why I established collaborative efforts with experts in microbiology, chemistry, nutrition and experimental and clinical science to seek, develop and test probiotics both experimentally and clinically, which could be expected to constitute powerful tools to prevent sepsis of various kinds.
Interdisciplinary collaboration in the early 1990’s lead to the identification of some L plantarum strains that demonstrated strong anti-inflammatory capacities. L plantarum 299, later used together with oatmeal in a synbiotic composition (72-74), is produced and marketed by Probi AB, Lund, Sweden. I participated heavily in this program until 1999, when I decided to re-direct my interest towards development and studies of a more complex synbiotic composition, designed not only to supplement four newly identified bioactive LABs in combination but also four different prebiotic fibers, already known for their strong bioactivity. Our aim was to provide this composition in much larger doses than was the practice at that time. Furthermore, knowing that most of the important LABs rarely exist in the microbiota of Westerners encouraged us to seek potent probiotic bacteria normally growing on plants instead of selecting bacteria normally found in human microbiota.
Since 1999, all my efforts in this field have concentrated on a four LAB/four fiber composition, consisting of either a mixture of 4×1010 (40 billion LAB, Standard version - Synbiotic 2000™) or a mixture of 1011 (400 billion Forte version - Synbiotic 2000 Forte™) based on the following four LAB: Pediococcus pentosaceus 5-33:3, Leuconostoc mesenteroides 32-77:1, Lactobacillus paracasei subsp paracasei 19, and Lactobacillus plantarum 2,362 in combination with 4×2.5 g of each of the following four fermentable fibres: betaglucan, inulin, pectin and resistant starch, in total 10 gr of prebiotic fibers per dose (75,76), a formula that is currently a product of Synbiotic AB, Sweden.
Perioperative prophylaxis in elective surgery
L plantarum 299 in a dose of 109 plus a total of 15 gram of oat and inulin fibers was tried, under research condition, in patients undergoing extensive abdominal surgical operations. The patient were mainly derived from those undergoing liver, pancreatic and gastric resections, equally distributed between three groups and supplemented with either: (I) live LAB and fiber, (II) heat-inactivated LAB and fiber, and (III) standard enteral nutrition (77). Each group comprised 30 patients. The 30-day sepsis rate was 10% (3/30 patients) in the two groups receiving either live or heat-inactivated LAB, compared to 30% (9/30 patients) in the group on standard enteral nutrition (P=0.01) [270]. The largest difference was observed in incidence of pneumonia: Group 1, 2 patients; Group 2, 1 patient; Group 3, 6 patients. The beneficial effects of treatment were seemingly most pronounced in gastric and pancreatic resections; the sepsis rate being: Group 1, 7%, Group 2, 17% and Group 3, 50%. The same pattern was observed for non-infectious complications: Group 1, 13% (4/30) Group 2, 17% (5/30); Group 3, 30% (9/30). The supply of antibiotics to Group 1 was significantly less (P=0.04) than to the other two groups, with the mean length of antibiotic treatment also considerably shorter: Group 1, 4±3.7 days; Group 2, 7±5.2 days; Group 3, 8±6.5 days.
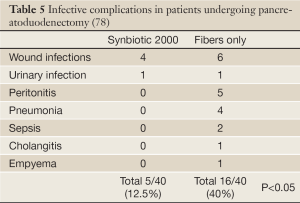
In a prospective, randomized, double-blind trial 80 patients undergoing pylorus-preserving pancreatoduodenectomy (PPPD) received, twice daily, either Synbiotic 2000TM (2×40 billion LAB, i.e. 80 billion LAB per day) or only the fibers in composition from the day before surgery and during the first seven postoperative days (78). A highly significant difference in infection rate (P=0,005) was observed as only 5/40 patients (12.5%) in the Synbiotic 2000-treated group suffered infections (4 wound and one urinary tract infection) vs. 16/40 (40%) in the fiber-only group (6 wound infections, 5 peritonitis, 4 chest infections, 2 sepsis, and one of each of urinary tract infection, cholangitis and empyema) - Table 5. The number of infecting microorganisms were also statistically and significant reduced - see Table 6. Statistically significant differences between the groups were also observed regarding the use of antibiotics (mean: Synbiotic 2000; 2±5 days, Only-fibers; 10±14 days) (78).
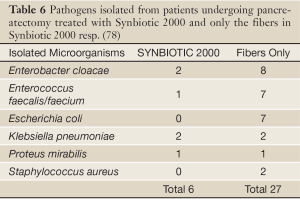
Full Table
In another randomized controlled study 45 patients undergoing major surgery for abdominal cancer were divided into three treatment groups: (I) enteral nutrition (EN) supplemented with Synbiotic 2000 (LEN), (II) EN supplemented with only the fibers in the same amounts (20 g) (20 g) as in Synbiotic 2000™ (FEN) and (III) Standard parenteral nutrition (PN). All treatments lasted for 2 preoperative and 7 days postoperative days. The incidence of postoperative bacterial infections was 47% with PN, 20% with FEN and 6.7% with LEN (P<0.05). The numbers of infecting microorganisms were also statistically and significantly reduced - see Table 7. Significant improvements were also observed in prealbumin (LEN, FEN), C-reactive protein (LEN, FEN), serum cholesterol (LEN, FEN), white cell blood count (LEN), serum endotoxin (LEN, FEN) and IgA (LEN) (Han Chun Mao, personal information).
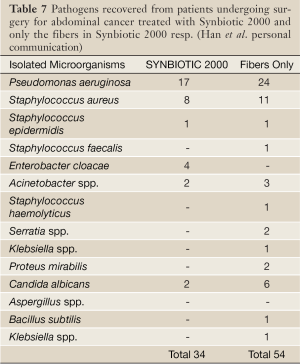
Full Table
Perioperative prophylaxis in liver transplantation
A prospective, randomized, study in 95 liver transplant patients supplemented L plantarum 299 in a dose of 109 plus 15 gram of oat and inulin fiber (79). Three groups of patients were studied: (I) selective digestive tract decontamination (SDD) four times daily for six weeks, (II) L plantarum 299 (LLP) in a dose of 109 plus 15g of oat and inulin fibres supplied postoperatively for 12 days, and (III) identical to group 2 but with heat-killed L plantarum 299 (HLP). Identical enteral nutrition was supplied to all patients from the second postoperative day. The numbers of postoperative infections were SDD 23, LLP 4 and HLP 17. Signs of infections occurred in SDD 48% (15/32), in LLP 13% (4/31), P=0.017 nd HLP 34% (11/32) respectively. The most dominant infections were cholangitis (which occurred: SDD 10, LLP in 2, and HLP in 8) and pneumonia (which occurred: SDD in 6, in LLP in 1, and HLP in 4). There was a statistically significant reduction in the numbers of infecting microorganisms, the most often isolated microbes being Enterococci and Staphylococci. Patients requiring haemodialysis were SDD: 8; LLP: 2 and HLP: 4 and the number of re-operations SDD: 6; LLP: 4 and HLP: 2 respectively. There were no deaths. The stay in ICU, the hospital stay and length on antibiotic therapy was shorter in the LLP group, but did not reach statistical significance. The CD4/CD8 ratio was also higher in the LLP group compared to the other two groups (P=0.06).
In a subsequent study, 66 human orthotopic liver transplant patients were randomized to either receive Synbiotic 2000 or only the fibers in Synbiotic 2000. The treatment was started on the day before surgery and continued for 14 days after surgery. During the first postoperative month only one patient in the Synbiotic 2000-treated group (3%) show signs of infection (urinary infection) compared to 17/33 (51%) patients in those supplemented with only the four fibers (80). Only one infecting organism was cultivated in the Synbiotic-treated group, which was shown to be Enterococcus fecalis, in contrast to seventeen organisms in the fiber- only treated group - see Table 8. The use of antibiotics was on average 0.1±0.1 d in the Synbiotic-treated patients and 3.8±0.9 d in the fiber- only treated group (80).
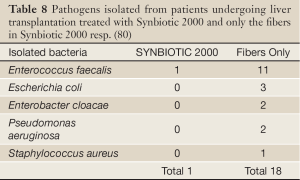
Full Table
Early treatment in major trauma
Two prospective randomized trials with Synbiotic 2000™ and Synbiotic 2000 Forte™ respectively were undertaken. In the first study (81) the patients were randomly allocated into 4 groups in order to compare the efficacy to prevent both total infections and particularly chest infections of the three commercial nutrition solutions: Group A: Alitraq (Abbott-Ross, Abbott Park, IL) 5.25 g protein, 16.5 g carbohydrate, 1.55 g fat and 1.55 g glutamine, 446 mg arginine, 154 mg α-linolenic acid per 100 mL. The osmolality is 480 mOsml/L. Group B: Nova Source (Novartis Medical Nutrition, Basel, Switzerland) 4.1 g protein, 14.4 g carbohydrate, 3.5 g fat, 2.2 g fermentable fibers as fermentable guar gum per 100 mL. The osmolality is 228 mOsm/L. Group C: Nutricomp peptide (B. Braun, Melsungen, Germany) 4.5 g hydrolyzed protein, 16.8 g carbohydrate, 1.7 g fat per 100 mL. The osmolarity is 400 mOsm/L. A fourth solution, Nutricomp standard, was chosen to be tried in combination with Synbiotic 2000- Group D: Nutricomp standard (B. Braun, Melsungen, Germany) supplemented with Synbiotic 2000, Nutricomp standard contain 3.7 g protein, 13.7 g carbohydrate, 3.3 g fat per 100 mL. The osmolarity of this solution is 240 mOsm/L. One sachet of Synbiotic 2000 was added to the solution before delivery to the patients (81). Nova Source and Nutricomp peptide did not reduce the levels of proinflammatory cytokines while Alitraq and Nutricomp standard + Synbiotic 2000 significantly down-regulated Il-6, but not Il-8 and TNF- α. Table 9 lists the total number and number of chest infections associated with the various nutrition solutions. The group containing Synbiotic 2000 demonstrated significant reductions in both total infections as in chest infections; the total number of infections being reduced with about two thirds and the number of chest infections being reduced to about half (81) - see further Table 9.
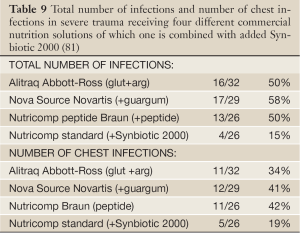
Full Table
In the other study (82-84) 65 polytrauma patients were randomized to receive once daily, for 15 days following major trauma, either Synbiotic 2000 Forte (400 billion LAB + 10 gram of fibers, see above) or maltodextrine, as placebo. Significant reductions were observed between the groups in the number of deaths (5/35 vs. 9/30, P<0,02), severe sepsis (6/35 vs. 13/30, P<0.02), chest infections (19/35 vs. 24/30, P<0.03), central line infections (13/32 vs. 20/30, P<0.02), and ventilation days (average 15 vs. 26 days [66]) (82-84). A total of 54 pathogenic microorganisms were cultivated in the Synbiotic treated group compared to 103 in the maltodextrine group - see Table 10 (82-84). Repeat analyses also revealed that serum levels of endotoxin (LPS) were decreased and ‘time to bloodstream infection’ significantly prolonged in patients treated with Synbiotic 2000 Forte.
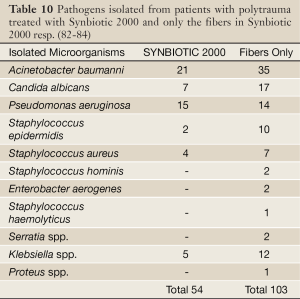
Full Table
Early treatment in severe acute pancreatitis
In a further study, patients with severe acute pancreatitis were randomized to receive either a freeze-dried preparation containing live L plantarum 299 in a dose of 109 together with a substrate of oat fiber or a similar preparation but heat-inactivated, administered daily through a nasojejunal tube for seven days (85). The study was concluded when, on repeat statistical analysis, significant differences in favour of one of the two groups were obtained. This occurred when a total of 45 patients had entered the study. 22 patients had, at that time, received treatment with live, and 23 with the heat-killed, L plantarum 299. Infected pancreatic necrosis and abscesses were seen in 1/22 (4.5%) in the live LAB group vs. 7/23 (30%) in the heat-inactivated group (P=0.023). The only patient in the lactobacillus group, who developed infection, a urinary infection, did so on the fifteenth day, i.e. at a time when he had not received treatment for eight days. The length of stay was also considerably shorter in the live LAB group (13.7 vs. 21.4 days) but the limited size of the material did not allow the statistical analysis to reach full significance (85).
Sixty-two patients with severe acute pancreatitis (SAP) (Apache II scores: Synbiotic 2000-treated 11.7±1.9, controls 10.4±1.5) were given either two sachets/day of Synbiotic 2000™ (2×40 billion LAB/day and totally 20 g fibers) or the same amounts of fibers (20 g) as in Synbiotic 2000™ during the first 14 days after arrival at the hospital (86). 9/33 patients (27%) in the Synbiotic 2000-treated group and 15/29 patients (52%) in the fiber-only treated group developed subsequent infections. 8/33 (24%) of the Synbiotic 2000-treated and 14/29 (48%) of the fiber-only treated patients developed SIRS, MOF or both (P<0.005). A total of seven pathogenic microorganisms were cultivated in the Synbiotic-treated group compared to seventeen in the fiber-only group (86) - see Table 11.
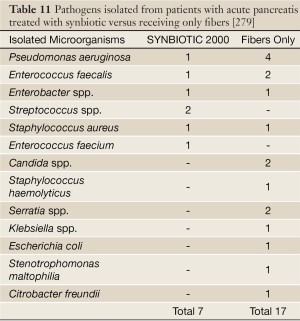
Full Table
In a third study (87) Synbiotic 2000 Forte was administered within 24-48 hrs of symptoms of sickness to patients with severe acute pancreatitis and compared to 32 patients on a control formula, the average volume and amount of calories being the same in the two treatment groups. The study demonstrated a lower infection rate (including pancreatic and peripancreatic necrosis); secondary infections (2 vs. 9, P=0.0001), septicemia (2 vs. 7, P=0.03), lower rate of surgical interventions, (3 vs. 12, P=0.005), shorter stay in ICU (8 vs. 16 days, P=0.05), shorter hospital stay (23 vs. 36 days, P=0.03) and reduced mortality (0 vs. 17 patients, P=0.02) (87).
Effects on “mind clarity” – encephalopathy
Patients with critical illness, as well as patients with chronic disorders such as liver cirrhosis and diabetes, frequently suffer a mild but sometimes severe confusion, which often has its origin in the gut (88). Increasing evidence suggest that probiotics, alone but also in combination with plant antioxidants and fibers, possess strong neuro-endocrine modulatory effects and can alleviate the effects of physical and mental stressors (89,90). We undertook some studies to explore the effects of Synbiotic in patients with liver cirrhosis and minimal encephalopathy (MHE) (91). Fifty-five patients with MHE were randomized to receive for 30 days: (I) Synbiotic 2000 (n=20), (II) the fibers in the composition alone (n=20), or (III) a placebo (n=15). All cirrhotic patients with MHE were found to have severe derangements of the gut micro-ecology and significant overgrowth of potentially pathogenic Escherichia coli and Staphylococcal species. Synbiotic treatment significantly increased the fecal content of non-urease-producing Lactobacillus species and reduced the numbers of potentially pathogenic micro-organisms. The treatment was also associated with a significant reduction in endotoxemia and in blood ammonia levels. A documented reversal of MHE was obtained in half of the treated patients, while the Child-Turcotte-Pugh functional class improved in about 50% of cases (91). Treatment with fermentable fibers alone also demonstrated substantial benefits in a proportion of patients.
In a second study, 30 cirrhotic patients were randomized to receive either Synbiotic 2000 or placebo for only 7 days (92). Viable fecal counts of Lactobacillus species, Child-Pugh class, plasma retention rate of indocyanine green (ICGR15), whole blood tumour necrosis factor alpha (TNF-α) mRNA and interleukin-6 (IL-6) mRNA, serum TNF-α, soluble TNF receptor (sTNFR)I, sTNFRII and IL-6 and plasma endotoxin levels were measured, pre- and post-treatment. The treatment with Synbiotic 2000 was associated with significantly increased fecal lactobacilli counts and significant improvements in ICGR15 and Child-Pugh class. Significant increases in whole blood TNF-α mRNA and IL-6 mRNA, along with serum levels of sTNFRI and sTNFRII, were also observed and TNF-α and IL-6 levels correlated significantly, both at baseline and post-Synbiotic treatment. Synbiotic-related improvement in ICGR15 was accompanied by significant changes in IL-6, both at mRNA and protein levels, but this was unrelated to levels of plasma endotoxin. No significant changes in any parameter were observed following placebo treatment. This study concluded that even short-term synbiotic treatment significantly modulated gut flora and improved liver function in patients with cirrhosis (92). Minimal encephalopathy is common not only in liver cirrhosis but is also seen in other chronic diseases such as diabetes. The observations in patients with liver cirrhosis gives hope that Synbiotic treatment may also be effective in other chronic diseases. See also (93).
Effects in HIV
It is well documented that disturbance of the microbiota occur early in HIV-1 infection, which leads to greater dominance of potential pathogens, reduced levels of Bifidobacteria and lactobacillus species and increasing mucosal inflammation. Current and emerging studies support the concept that probiotic bacteria can provide specific benefit in HIV-1 infection. It was not until Brenchley et al. in 2006 identified translocation of microbes or microbial products without overt bacteremia, as a major cause of systemic immune activation in HIV-1 and SIV infection (94), that a greater interest in bio-ecological treatment emerged.
Impairment of the GI tract in HIV-positive patients is already present in the early phases of HIV disease and is associated with elevated levels of intestinal inflammatory parameters and definite alterations in the gut commensal microbiota, confirming a possible correlation between intestinal microbial alteration, GI mucosal damage, and immune activation status, further confirming that alterations at the GI-tract level are a key factor in the pathogenesis of chronic HIV infection (95). The findings, in a recent study, of fairly mild changes in microbiota of HIV-infected individuals, before initiation of pharmacological treatment, might suggest that the later observed more profound alterations in microbiota could be pharma-induced, as only a trend to a greater proportion of Enterobacteriales compared to control subjects (P=0.099) were observed, despite the significant negative correlations between total bacterial load and duodenal CD4+ and CD8+ T-cell activation levels (96). As pointed out in a recent review, current and emerging studies appear to support the concept that probiotic bacteria can provide specific benefit in HIV-1 infection. Probiotic bacteria have proven active against bacterial vaginosis in HIV-1 positive women and have enhanced growth in infants with congenital HIV-1 infection (97). Probiotic bacteria may also stabilize CD4+ T cell numbers in HIV-1 infected children and are likely to have protective effects against inflammation and chronic immune activation of the gastrointestinal immune system [288].
Recent studies at least partly support the assumption that L rhamnosis GR-1 and L Reuteri RC-14 tend to increase the probability of a normal vaginal flora (odds ratio 2.4; P=0.1) and significantly increase the probability of a beneficial vaginal pH (odds ratio 3.8; P=0.02) at follow-up (98,99). However, later attempts using probiotic yoghurts have proven less successful (100). In a recent pilot study 38 women with HIV, taking highly active antiretroviral therapy (HAART), were supplemented with Synbiotic 2000 Forte orally for 4 weeks (101). In a surprising and very encouraging observation, the supplemented formula showed ability, despite heavy pharmaceutical treatment, to survive during the passage through the GI tract, and also the ability to colonize the gut and contribute to a significantly elevated level in the stool of the supplemented LAB group. The T-cell activation phenotype was altered by exposure to the Synbiotic formula and was accompanied by a slightly elevated HLA-DR expression of a minor population of CD4+ T-cells, which normally lack expression of HLA-DR or PD-1. These significant changes occurred in the context of unaltered microbial translocation, as measured by plasma bacterial 16S ribosomal DNA (101). It is especially encouraging that the LAB supplemented with Synbiotic 2000, despite heavy medication/highly active antiretroviral therapy (HAART), were able to colonize the gut and seemingly, at least slightly, improve immune functions. Hopefully, significantly more pronounced positive effects will be obtained the day we are ready to try eco-biological treatment, not only as complementary treatment but as an alternative to pharmaceutical treatment.
It is all about inflammation
Inflammation, an essential component of immune-mediated protection against pathogens and tissue damage, and uncontrolled immune responses, will commonly, especially in Westerners, institute a state of chronic inflammation, which will occur when immune response are activated despite the absence of ‘danger’ signals, fail to fully turn-off despite elimination of danger signals and/or fail to completely clear such signals. Numerous factors, in addition to genetic predisposition, trauma and various stress factors (physical and emotional) are known to contribute to increased discrete and long-lasting inflammation, among them age, diet and medications.
Studies of human gene-related inflammation suggest that, of the approximately 25,000 human genes, approximately 5%, or some 1,200 genes, are involved in inflammation (102-104). It is increasingly understood that the human genome in itself will only explain a minority of chronic diseases, far less than changes in lifestyle, food habits and social behavior, factors which seem to have a dominating impact on human health. Clearly, the molecular mechanisms linking environmental factors and genetic susceptibility was first envisioned after the recent exploration of the, until recently hidden, source of genomic diversity, i.e. the metagenome with its more than 3 million genes (105). Although the mechanisms behind the metagenome-associated low-grade inflammation and the corresponding immune response are not yet fully understood, there is no doubt that the metagenome has a dominating influence on altered body functions such as adipose tissue plasticity and diseases such as hepatic steatosis, insulin resistance and cardiovascular diseases, but also on disorders such as autoimmune diseases including rheumatoid arthritis, gastrointestinal and neuropsychiatric diseases and on development and progress of a number of cancers (106), as well as many other chronic disorders. When disease exacerbations occur, in trauma or in critical illness, the normally silent or discrete inflammation turn into a storm (107) as experienced in systemic inflammatory response syndrome (SIRS) and multiple organ failure (MOF) (108). In many severe conditions like MOF and SIRS components of cytokine-induced injury might be more damaging than the initial cause/trauma/early invasion of micro-organisms in themselves. Inflammatory cytokines, such as TNF alpha and IL-1β, released by these events will destabilize endothelial cell-cell interactions and cripple vascular barrier function, producing capillary leakage, tissue edema, organ failure, and sometimes death (108).
Cytokine-inhibition, pharma and/or probiotics?
Inflammation is, as discussed above, extraordinarily complex. In rheumatoid arthritis (RA) for example, the joints are rich in cytokine-secreting cells containing a wide range of effector molecules including pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α and IL-18, chemokines such as IL-8, IP-10, MCP-1, MIP-1 and RANTES, MMPs such as MMP-1, -3, -9 and -13 and metabolic proteins such as Cox-1, Cox-2 and iNOS, which interact with one another in a complex manner that is thought to cause a vicious cycle of pro-inflammatory signals resulting in chronic and persistent inflammation (109,110). NF-КB is increasingly suggested to be the master regulators of inflammatory cytokine production in RA. These mediators are also involved, although not in an identical manner, in other autoimmune disorders such as inflammatory bowel diseases (111). This knowledge has led to development to a new generation of pharmaceutical drugs, generally referred to as biological, designed to inhibit the crucial mediators of pro-inflammatory signals and subsequent abnormal immune response. A whole series of revolutionary new drugs such as anti-TNF-α, anti-IL-1β, anti-HER2 etc are already successfully tried and new drugs such as antibodies targeting IL-12/IL-23 pathways, IFN-γ, IL-17A, IL-2 and IL-6, and also inhibitors of NF-КB more or less extensively tried in a variety of chronic inflammatory and autoimmune diseases. Some of these have already demonstrated initially promising result, while other treatments such as administration of the regulatory cytokines IL-10 and IL-11 have failed to induce reproducible clinical effects (111). Significant benefits in quality of life and tissue/organ healing are encountered in at least more than 50% of treated cases. These drugs are generally tolerated well, but adverse events such as infections including reactivating tuberculosis, tumours such as lymphomas and demyelinating diseases and infusion reactions are sometimes evident. These changes must be regarded as acceptable as long as they are used in diseases that have proven to be refractory to all other treatments but may be an issue when, as increasingly suggested, they are tried in early stages of diseases, as happened after widening indications for statins (112).
Single target or multitarget treatment?
Most biologicals are designed to target single molecules, those regarded as mainly responsible for the etiology of disease, even if they in reality actually affect several other molecules. There are also indications that sometimes the results of selective targeting may be short-lasting and that the inflammation sooner or later will find other pathways and the disease consequently continue to progress. In most diseases a large number of pro-inflammatory abnormalities are observed; for these broad-spectrum e.g., ecobiological treatments might offer a good, and sometimes better, solution than single-gene targeting biological treatment - see further below.
It is unfortunate that no studies thus far have addressed the effects of the biologicals on microbiota and leakimg barriers. Until done, one must assume that these drugs have the same devastating effects on microbiota and barrier efficacy as other drugs. Plant-derived mediators, or phytochemicals, such curcumin, resveratrol, genistein etc, (see further above) and plant fibers, particularly prebiotic fibers, and probiotic bacteria, which may be termed ‘eco-biologicals’ possess, can be expected, alone or in combination, to have the same molecular functions as biologicals - although much weaker - but also without known adverse effects. Compounds officially classified as GRAS, generally considered as safe, should be considered where the main indications are prevention, early in disease treatment but also where used as palliative treatment particularly in children and the elderly - Figure 2.
Asian foods associated with less disease
Several population-based studies indicate that people in Southeast Asian countries have a much lower risk of developing colon, gastrointestinal, prostate, breast, and other cancers than do their Western counterparts. They also have significantly reduced incidence of other chronic diseases such as coronary heart diseases, neuro-degenerative diseases, diabetes, inflammatory bowel diseases, etc. A significantly lower morbidity and mortality is also often observed in countries in this part of the world. Much supports that morbidity and mortality in critically ill is also significant reduced in Southeast Asian countries compared to the West. It is also likely that the frequent use in these countries of dietary constituents containing antioxidant/chemo-preventive molecules (see Figure 3), as is the case with garlic, ginger, soybeans, turmeric, onion, tomatoes, cruciferous vegetables, chilies, and green tea and many others may play an important role in protection from cancers, and other diseases, and poor outcome in critical care, as these dietary agents have the ability to suppress transformative, hyper-proliferative, and inflammatory processes (113).

Present knowledge, as obtained from extensive research, especially in most recent years suggests that good health and well-being, in addition to regular physical exercise, good sleep and control of stress/spiritual harmony is strongly associated with the food we eat, and its influence on the body, particularly the microbiota. It is unfortunate, that the sickest patients are more or less in constant stress, cannot exercise and receives the worst nutrition possible. To change these conditions should be a highly prioritized challenge for the future.
Seven NOs and three YES!
I suggested in a recent review ten important principles to be met in our striving for an optimal nutrition (114):
-Restrict intake of insulinogenic, IGF1-rich or IGF1-stimulatory and Toll-stimulatory foods such as refined carbohydrates; cereals, bread, sweats, cookies, rice, pasta, cooked tubers incl. potatoes, foods, which are absorbed high in the small intestine and of minimal benefit to microbiota.
-Restrict daily intake of fructose to below 25 gram a day.
-Restrict intake of dairy products especially butter, cheese and milk powder, rich in saturated fats, hormones and growth factors such as IGF1, and also meat intake, especially inflammation-inducing, processed and cured meat such as bacon and sausages. See further (115-117).
-Restrict/eliminate intake of foods, which are heated above 100 oC known to be rich in the inflammation-inducing molecules AGEs and ALEs, and particularly foods heated above 130 oC, as foods with increased temperature becomes increasingly rich in pro-inflammatory and carcinogenic substances such acrylamide and heterocyclic amines e.g., fried and grilled foods and toasted and high-temperature baked breads. See also (115-117).
-Restrict exposure to microbe-derived highly inflammation-inducing endotoxin, especially rich in meat hung for several days, hard cheeses, pork and ice-creams.
-Restrict, eventually eliminate intake of foods rich in proteotoxins such as casein, gluten and zein.
-Restrict intake of chemicals including pharmaceutical drugs to only what is absolutely necessary as most likely most chemicals are detrimental to microbiota.
-Increase dramatically intake of fresh and raw greens, fresh spices and vegetables, rich in antioxidants, fibers, minerals and nutrients, but also inflammation-controlling factors such as curcumin, resveratrol and many other - see Figure 3.
-Increase/favour intake of ancient anti-oxidant-rich, high fiber, low-calorie containing grains such as buckwheat, amaranth, chia, lupin, millet, quinoa, sorghum, taro, teff etc, and also intake of beans, peas, chickpeas, lentils, nuts and almonds. See Table 12.
-Supplement of large doses of vitamin D and omega fatty acids, both of special importance for control of inflammation and for function of microbiota. If sick -do also supplement pro-/synbiotics, but only brands with documented clinical effects.
Nutrition of the sick made to mimic healthy foods for the healthy
If supplies of health-supporting nutrients are important to the already healthy individuals, it is no doubt even more important in the already ill patients. It is increasingly recognized that today’s artificial foods on the market, everything from pet foods, baby formulas and clinical nutrition solutions are far from meeting such requests, and instead often contribute to development of or aggravating disease. It is especially so with present clinical nutrition solutions, which often are produced from cheap raw materials such as evaporated milk or milk powder and similar instead of made to mimic natural healthy foods. Of great importance is the choice of sources and amounts of energy and the choices of proteins, carbohydrates and fats in the various compositions, as well as the content of micronutrients. Furthermore, all patients are not similar and have not similar needs, requests consequently made for specific formulations to be used in premorbid conditions such as diabetes (119) and obesity (120). As currently, approximately 230 more or less different enteral feeding formulas and nutrition supplements are commercially available (120), there should be place for mot specialized and adapted to need formulas.
Source of protein critical
The protein needed in the formula can be obtained from a large variety of sources, animal proteins as well as plant-proteins. Animal proteins commonly used in clinical nutrition formulas are usually milk-based and consist either in casein (80% of milk proteins), whey (20% of milk protein) or a mixture of both. Caseins are colloidal aggregations of as1-, as2-, b- and k-caseins and whey comprises fractions of β-lactoglobulin, α-lactalbumin, lactoferrin, various immunoglobulins, proteose-peptone and serum albumin. All proteins, irrespective of source seem to be effectively metabolized in the small intestine. A recent study in healthy humans suggests that proteins that pass the terminal ileum are already at that stage dominated by bacterial protein; ~60% bacterial protein, ~15% mucin protein, ~7% soluble-free protein, and ~5% protein from intact mucosal cells, indicating a substantial microbial activity already within the human distal small intestine (121).
Limited data seems to be available to specifically support the choice of these protein sources. The biological effects vary between different types of proteins. Some proteins such as Alpha-lactalbumin, gelatin and gelatin enforced with tryptophan are reported to provide higher degree of satiety (app 40%) than any of the commonly used proteins in nutrition formulas, others like casein, soy, whey, whey enforced with glycomacropeptide (GMP) reported to induce a significant reduction in energy intake (app 20%) (122). Different types of proteins are also absorbed differently and will consequently influence insulin response differently, significant differences observed between fast-absorbing whey and soy and slow-absorbing casein (123).
Whey-based proteins have advantages over casein-based
Enteral nutrients (EN) are known to potentiate the action of glucagon-like peptide-2 (GLP-2)s a nutrient-regulated intestinotrophic hormone, derived from proglucagon in the distal intestine and to reverse mucosal hypoplasia induced among others by exclusive parenteral nutrition (PN). When the ability of different proteins; casein, hydrolyzed soy, whey protein concentrate (WPC), and combined hydrolyzed WPC and casein were tried in an animal study only whey protein, but not the others demonstrated potential to enhance the ability of GLP-2 to reverse mucosal hypoplasia and increase mucosal cellularity as well as the absorptive surface area (124). Emerging evidence support that consumption of different types of proteins will have different stimulatory effects on the amplitude and possibly also duration of muscle protein synthesis after feedings-being greater after whey or soy protein consumption than after casein, both at rest and after exercise (125).
The capacity of the proteins to inhibit systemic inflammation is of special importance. When in an animal study the ability of three different protein sources to resist LPS-induced systemic inflammation was studied; a whey-based formula (Peptaman AF, Nestlé Healthcare Nutrition) demonstrated to be significantly superior to a commercial casein-based diet (Promote® Abbott) and a standard soy-based diet high in cysteine and crude fiber (44). Another animal study demonstrated that supply of whey protein diet to exercise-trained rats resulted in comparison to casein-supplied and soy supplied animals significantly higher levels of liver glycogen, as a result of effects on regulation of rate limiting glycolytic and gluconeogenic enzyme activities but also due to activation of glycogenesis from alanine via alanine amino-transferase (126). A recent animal study suggests that whey proteins might also possess significant anti-inflammatory abilities; significant decreases in levels of, IL-1β, TNF-α, IL-6, IL-4, malondialdehyde (MDA), nitric oxide (NO) and reactive oxygen species (ROS), in neutrophil infiltration as well as in wound healing time was observed (127).
Clearly use of whey proteins seem to have advantages particularly over casein, but most likely also over soy-derived proteins. However, it is disturbing that despite the wide use of supplementation of bovine milk-derived proteins convincing evidence from human studies are still largely lacking and when existing not providing convincing evidence for widespread clinical use. A recent study in young boys fed casein, demonstrated a significant 15% (P<0.0001) increase pro-inflammatory IGF1/s but no changes in fasting insulin (P=0.36), while boys fed whey instead had a 21% (P=0.006) increased fasting insulin, and no change in IGF-1 (P=0.27) (128). A critical review examined twenty-five recently published intervention trials examining chronic and/or acute effects of whey protein supplementation on lipid and glucose metabolism, blood pressure, vascular function and on the musculoskeletal system, concluding that although whey protein may affect glucose metabolism and muscle protein synthesis, but the evidence for a clinical efficacy is not strong enough to make any final recommendations for its use in humans (129). A recent study (130) demonstrated a great potential of feeding rice protein to improve oxidative stress primarily through enzymatic and non-enzymatic antioxidative defense mechanisms; the total antioxidative capacity (T-AOC), mRNA levels of glutamate cysteine ligase catalytic subunit (GCLC) and glutamate cysteine ligase modulatory subunit (GCLM) mRNA levels, antioxidative enzyme activities (T-SOD and CAT) and glutathione metabolism related enzyme activities), γ-glutamylcysteine synthetase (γ-GCS), glutathione S-transferase (GST), glutathione reductase (GR) and glutathione peroxidase (GSHPx) were all effectively stimulated by feeding rice protein compared to casein. Furthermore rice protein feeding did significantly reduce the hepatic accumulation of of markers of inflammation; malondialdehyde (MDA) and protein carbonyl (PCO) (130).
Pseudocereals - promising alternative protein source
Pseudocereals such as amaranth, quinoa and buckwheat with their somewhat higher content of protein than for example wheat, definitely higher fat content (amaranth and quinoa) favorable content of dietary fibres (especially amaranth and buckwheat) might represent promising alternatives - see also Table 12 (118). Furthermore the composition of fat in amaranth, quinoa, buckwheat and wheat seeds are favorable- Oleic acid (C18:1): amararant 23.7 quinoa 26.7 buckwhear 33.6 wheat 13.2 Monounsaturated fatty acids: amaranth 23.9 quinoa 28.1 buckwheat 34.7 wheat 13.4 - all g/100 g. Cereal, pseudo-cereal and leguminous flours are known to be very rich in amino acids (118), with a pattern much similar to the human muscle - especially buckwheat, and constitute excellent substrates for biosynthesis of γ-aminobutyric acid (GABA), known to effectively reduce inflammation and prevent tissue injury (131) and to exhibit strong calming effects; effective against fear, anxiety, depression, headache and other mental conditions [see for example (132)]. Strains of Lactobacillus plantarum and Lactococcus lactis have been proven effective to ferment cereal, pseudo-cereal and leguminous flours and effectively release both amino acids as well as GABA. A blend of buckwheat, amaranth, chickpea and quinoa flours in the proportions 1:1:5.3:1 was recently when fermented with L plantarum C48 demonstrated to produce high concentration of free amino acids (ca. 4,467 mg/kg) and GABA (504 mg/kg) (133,134). It is my opinion that it should be possible in the future using similar technology to produce clinically efficient ingredients for new dramatically different clinical nutrition formulations.

Full Table
Sources of fats for clinical nutrition
Mainly three other fat emulsions, apart from the commonly used soybean oil (SO)-based emulsions, have been used in parenteral nutrition formulas; medium-chain triglycerides (MCTs), olive oils (OOs), and fish oil (FOs). Enteral nutrition have to large extent been done to mimic these solutions. It is possible, as all these oils are metabolized via different pathways, that they may affect different functions in the body; either induce or inhibit inflammation in the body or reduce or enhance immune suppression. A recent small study compared 12 patients receiving a mixture of soybean and medium-chain triglyceride oils (group A) with 18 patients receiving a fat emulsion with part of the lipid replaced by fish oil (group B) reporting a trend toward reduced serum inflammatory cytokines in group B vs. group A with significant differences regarding interleukin (IL)-1, IL-8, and interferon (IFN)-γ on postoperative day 4 (P<0.05) and IL-1, IL-8, IFN-γ, IL-6, and tumor necrosis factor-α on postoperative day 7 (P<0.05), but more interestingly, although not statistically significant, a reduction in postoperative liver dysfunction (B vs. A: 33% vs. 50%) and infection rate (B vs. A: 28% vs. 42%) (135) was observed. However other similar recent studies have been unable to verify any significant difference between such groups (136).
Omega-3- based emulsions receive increasing interest
Lipid mediators derived from the n-3 fatty acids eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) are increasing used in clinical nutrition to reduce systemic inflammation. Intercellular mediators such as protectins and resolvins, known to either protect from or induce resolution of inflammation, are enzymatically generated from n-3 fatty acids in the body [see further (137)]. Experimental data suggest that n-3 fatty acids may improve acute lung injury and sepsis. Application of n-3 fatty acids to patients undergoing major surgery seems to provide some although not dramatically beneficial effects such as reduction of length of stay and infectious complications (138,139). Recent human studies, however, have been unable to support such conclusions, at least in severely critically ill patients on mechanical ventilation. A recent multicenter study (140) involving 272 adults provided within 48 hours of developing acute lung injury and requiring mechanical ventilation enteral supplementation of n-3 fatty acids, γ-linolenic acid, and antioxidants. Despite an 8-fold increase in plasma eicosapentaenoic acid levels, patients receiving the n-3 supplement had compared to iso-caloric controls only slightly but statistically significantly fewer ventilator-free days (14.0 vs. 17.2; P=0.02) (difference, –3.2 [95% CI, –5.8 to –0.7]) and intensive care unit-free days (14.0 vs. 16.7; P=0.04). Patients in the n-3 group also had fewer non-pulmonary organ failure-free days (12.3 vs. 15.5; P=0.02). Sixty-day hospital mortality was 26.6% in the n-3 group vs. 16.3% in the control group (P=0.054), and adjusted 60-day mortality was 25.1% and 17.6% in the n-3 and control groups, respectively (P=0.11). Use of the n-3 supplement resulted also in more days with diarrhea (29% vs. 21%; P=0.001) (140).
Sixty-four adult patients undergoing surgery for gastrointestinal diseases were randomly assigned to receive isocaloric and isonitrogenous total parenteral nutrition with either an ω-3 fatty acid-enriched emulsion (Lipoplus, n=32) or medium-chain triacylglycerols/long-chain triacylglycerols (Lipofundin, n=32) for 5 d after surgery (141). Total bilirubin decreased faster in the ω-3 fatty acid-enriched group (P=0.017), and the so called activated partial thromboplastin time was significantly prolonged between days 1 to 3 (P=0.002) than in the other group. Although no differences were observed in C-reactive protein, interleukin (IL)-1, IL-8, IL-10, vascular endothelial growth factor (VEGF), and distribution of the T-cell subpopulation between the two groups, significant decreases in IL-6, tumor necrosis factor-α, and nuclear factor-κB, occured in parallel to significant increases in leukotriene B5/leukotriene B4 in the fishoil-treated group (141).
Olive oil-enriched emulsions might have their advantages
Other sources of fat for clinical nutrition have been tried. A recent study compared in critically ill adults parenteral administration of an olive oil-based lipid emulsion with a standard soybean oil-based lipid emulsion but found no differences in; rates of infectious and noninfectious complications, glycemic control, inflammatory and oxidative stress markers, and immune function (142). However, a recent prospective, randomized, controlled, crossover study done by the same group and focusing on vascular, metabolic, immune, and inflammatory effects of 24-h parenteral infusion of an olive oil-based emulsion (ClinOleic) report compared to a soybean oil-based lipid emulsion (Intralipid) definite advantages of the olive-oil based formula, as the soybean oil-based lipid emulsion increased blood pressure and impaired endothelial function, no such changes were seen with the use olive oil-based lipid emulsion or lipid-free enteral nutrition solutions (143).
Great need for new enteral nutrition formulas
The shift in clinical praxis from an almost totally parental nutrition dominated praxis to an enteral nutrition dominated praxis in post-surgical, post-trauma and critically ill patients has changed clinical praxis and improved the clinical outcome. However, the formulas for enteral nutrition, has not changed as could have been expected, they continue to look much as they are constructed for parenteral use. Although the knowledge about healthy and unhealthy foods has increased dramatically and eating habits at least among health-concerned individuals changed considerably in recent years, this information has not translated into a new generation of enteral nutrition formulas made to mimic healthy foods; rich in greens, never heated to high temperatures (low content of AGE and ALE), no ingredients of proteotoxins such as gluten, casein and zein etc. Greens and plants match human dietary needs closer than any other foods and there is strong evidence that they lead to a strong immune system, increased resistance to disease and profoundly stronger tissues, especially muscles. There are thousands of varieties of greens and plant foods and each one has its’ own unique set of nutrients, which is why it is important with a great variation in food sources. Green smoothies rich in raw foods; greens (liquidized salads), avocado, banana, carrots, fruits etc as well as fresh raw-food based soups the Spanish Gazpacho could constitute interesting alternative, if hygienic conditions could be solved.
Avocado rich in antioxidants
The increased interest in recent years in olive oil as a source of healthy fats is interesting but also other fat sources such as avocado, coconut and red palm tree fruits, the last two demonized in the past due to their high content of saturated fat, but today increasing recognized for its health-promoting ingredients and clinical effects. The avocado fruits are especially rich in monosaturated fats and also vitamins E and C, carotenoids and sterols, important ingredients that possess antioxidant and radical scavenging activities, and shown to often be deficient in people living in Western Societies. It is also rich in lutein, alpha-carotene, beta-carotene, neoxanthin, violaxanthin, zeaxanthin, antheraxanthin as well as chlorophylls, and pheophytins. Supply of avocado or avocado oil has been shown to increase the uptake of carotenoids several-fold; lutein 5 times, alpha-carotene 7 times and beta-carotene 15 times (144). Avocado oil has been shown to reduce inflammation and protect tissues from destruction, especially observed on the musculoskeletal system (145).
Red palm oil rich in vitamin A, MUFAs and unsaturated fats
The lipid profile of palm oil has a near 1:1 ratio of saturated to unsaturated fatty acids. It is regarded as the richest natural source of dietary provitamin A carotenes, said to contain 15 times more provitamin A carotenes than carrots and 300 times more than tomatoes, one teaspoon of red palm oil per day enough to supply the recommended daily allowance (RDA) of vitamin A for children (146). It is also a most abundant natural source of vitamin E, α-tocotrienol, and α-iocotrienol, lycopenes, squalene, Co-enzyme Q10, and saturated and unsaturated fatty acids (known to maximize absorption of carotenoid anti-oxidants). It is also reported to possess unique tissue-protective capacity and especially neuro-protective properties. Palm oil–derived α-tocotrienol is reported to reach the brain in sufficient quantity to attenuate stroke-mediated neuropathy (147). Red palm oil with its high level of saturated fatty acid seems not, as earlier feared, to promote atherosclerosis and/or arterial thrombosis. The reason could be that most of the saturated fat in palm oil is medium-chain fatty acids (MCFA), which is absorbed directly into the portal vein, and rapidly transported to the liver for beta-oxidation, while long-chain fatty acids (LCFA),in the form of triacylglycerols, which dominate Western type foods, are absorbed via the intestinal lymphatic ducts and transported by chylomicrons through the thoracic duct and directly into the systemic circulation, a difference, which might explain the dramatically different effects on systemic inflammation and health (148).
Red palm oil (and coconut oil) effective against inflammation
As a matter of fact, red palm oil has been reported to reduce the risk of arterial thrombosis and/or atherosclerosis, to inhibit endogenous cholesterol biosynthesis, platelet aggregation, reduce oxidative stress and significantly reduce blood pressure. It is suggested that dietary red palm oil, consumed in moderation by animals and humans, have the ability to promote efficient utilization of nutrients, activate hepatic metabolism of drugs, facilitate haemoglobinisation of red blood cells and improve immune function (146). In large parts of Africa red palm oil is regarded as potent inhibitor of progress of HIV, but studies are lacking to support such a belief. However, a recent in vitro study on human monocytic cells confirm that red palm oil is an remarkably effective inhibitor of LPS-induced generation of NO, production of PGE2, stimulation of secretion of proinflammatory cytokines (TNF-α, IL-4, and IL-8), and expression of iNOS, COX-2, and NF-jB. The production of PGE2 and down-regulating of expression of COX-2 and iNOS occurred in a dose-dependent manner, observations supporting that the potent anti-inflammatory activity of red palm oil is associated with blockage of NF-КB activation and selective inhibition of COX-2 expression (149). The demonstration that red palm oil protects against ischemia and reperfusion injuries of the heart is most promising (150-152) and merits further investigations.
Medium-chain fatty acids have anti-infectious effects and do not produce resistance to pathogens
Capric acid (C10:0) and lauric acis (C12:0), both rich in vegetable such as coconut or palm oil, and the essential fatty acid, linoleic acid, (C18:2), especially rich in safflower oil, evening primrose oil and poppy- seed oil are all known for their powerful bactericidal effects on a large number of bacteria, viruses and funguses. There is growing evidence that the role of lipids in innate immunity is more important than previously realized. Common fatty acids and sphingolipids are involved in the physical barrier, permeability barrier, and immunologic barrier functions especially of the skin and mucosal surfaces. The antibacterial actions of FFAs are typically broad spectrum and of potencies comparable to natural antimicrobial peptides (AMPs). While FFAs are not as structurally diverse as the more widely studied AMPs, their importance in the human innate immune system is well-established, particularly in the defense of skin and mucosal surfaces [see further (153)]. Furthermore, the fact that resistant phenotypes as seen with conventional antibiotics do rarely exist with FFA treatment makes plant-derived FFAs an attractive anti-infectious alternative to be used both in medicine in general and in clinical nutrition in particular.
Special focus on fat metabolism in liver and pancreas disease
Modern man has much more fats in the abdomen than our Paleolithic fathers. The amount of fat in the abdomen can vary from a few milliliters in a lean subject to approximately 6 L in gross obesity (154), abnormal amounts of fat were not possible before the adventure of modern agriculture. Visceral adipocytes are, compared with subcutaneous fat cells, known to secrete much more free fatty acids, but also approximately three times as much of proinflammatory molecules such as IL-6 and PAI-1 per gram tissue (155). These observations explain not only the significantly higher risk of developing various chronic but also acute diseases and complications to invasive treatments in individuals with visceral obesity. The stress-induced load of mobilized fats and proinflammatory and procoagulant molecules from visceral adipocytes via the portal vein on the liver is much more than the liver can cope with, especially when functionally reduced by disease or surgery - a load, which often is increased by 1,000 times (155). Other organs, the brain, the lungs, the pancreas and the kidneys are also severely affected by this flood of proinflammatory factors. This development is serious in conditions where these organs are reduced in mass or functions, especially in conditions such as after extensive liver resection, liver transplantation and in patients with pre-existing reduced liver functions - already suffering from hepatic steatosis or liver cirrhosis. As a matter of fact, the accumulation of fat in the liver is heavy even when extensive liver resections are undertaken in young and healthy animals but dramatic in diseased humans (156,157). The increase of fat remains often for a month or more and is associated with a corresponding reduction in circulatory fats. It is advisable to strictly limit supply of nutritional fat as long as this condition exists.
Fatty livers are almost always associated with dysbiosis (158). It is promising that steatosis of the liver might be reduced by ecobiological nutrition; plant-derived antioxidants and nutrients (60,159,160) and particularly by supply of efficient probiotics (161,162). Recent experimental studies report significant hepato-protective effects of turmeric-derived curcumin observed; stabilization of redox state, reduced liberation of liver enzymes, and attenuated expression of pro-inflammatory cytokines after liver resection (163) and prevention of hepatic steatosis (164), a condition said to exist in every fourth Westerner, regularly consuming high-calorie-loaded Wstern foods.
Urgent need of new ecobiological formulas
Disciplines like immunotoxicology and immunopharmacology are still in their infancy. Much evidence exists to support that the drugs we often use in ICUs have strong and hitherto sometimes unrecognized, and most often ignored negative influences on the immune system and the sensitivity to inflammation and infection [see further (165)]. The increasing information about the damage that most pharmaceuticals produce to microbiota makes it urgent that priority be given to choice and use of pharmaceuticals, especially in the critically ill. It remains a great dilemma and a completely unsolved issue that the sickest and most demanding patients, not only are under constant mental and physical stress with no chance to physical exercise but also receive the most incomplete, sometimes dangerous nutrition, often containing ingredients documented to escalate inflammation and contribute to increased morbidity.
There is an urgent need of new completely different enteral nutrition formulas than the existing. The formulas presently used are only slightly different compared to the artificial formulas used for parenteral nutrition, made mainly to provide calories and support a favorable nitrogen balance. New insights necessitates formulas made mainly or entirely with the goal to restore homeostasis in inflammation and immune functions. The new science of nutrigenomics provides tools to identify the effects of various food ingredients and their effects on various genes, particularly those associated with inflammation and immune functions. Immediate attempts should be made to avoid nutrition formula ingredients such as long-chain saturated fats, trans-fatty acids, advanced glycation end products (AGEs) [see further (116)], hormones, and various stress molecules and sugars, particularly fructose. Future enteral nutrition formulas should be made to mimic normal food to the extent possible. Certainly, some already used regular foods, such as Mediterranean raw soups like the Spanish gazpacho and various other vegetable and fish soups, can be adapted for clinical use and provided, if necessary also by tube-feeding. Green leaves, fresh vegetable and fruit juices/smoothies can easily be adapted for clinical use and probiotic bacteria, prebiotic fibers, and plant antioxidants, various polyphenols, such as curcumenoids and resveratrol and similar molecules mixed into these solutions. I have only recently realized the special importance of green leaves with their, compared to the roots, low content of energy, but enormous richness in important nutrients, minerals, vitamins, antioxidants– some being hundreds or thousand times richer in the leaves - see Table 13 (166).
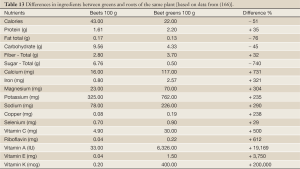
Full Table
Restoring microbiota - key to success
Studies in critically ill, especially those with systemic inflammatory reaction syndrome (SIRS) report severe dysbiosis with compared with healthy volunteers often 10,000 times fewer total anaerobes, including ‘‘beneficial’’ Bifidobacterium and Lactobacillus, and 100 times more "pathogenic" bacteria such as Staphylococcus bacteria. The content in the gut of organic acids in general but butyric and propionic acids in particular are severely reduced. Recent studies report the production of “mucosa-tightening” butyric acid as almost extinct (from 16.6±6.7 to 0.9±2.3) (65,167).
The incidence of organ failure and ICU mortality is reported to be significantly higher in patients with profound reductions in size and biodiversity of microbiota and especially when associated with a massive presence of enterococci and with the use of clindamycin (168). Information like this provide strong support to priority to efforts to prevent dysfunctioning microbiota, dysbiosis, and when needed - as it always is in in critically ill patients- strong and forceful efforts to restore homeostasis in microbiota, eubiosis, Recent cutting-edge results from the supplementation of synbiotics to postoperative and critically ill patients as reported above, support recommendations to routinely supplement specific LAB and fibers (synbiotics) to a wide group of patients undergoing major medical or surgical treatments or suffering from polytrauma or medical emergencies such as acute pancreatitis or myocardial infarction. For patients who cannot tolerate enteral feeding, administration of synbiotics by enemas with live LAB may be a treatment option. It is important to remember that most patients do not die of their disease but from a disordered physiology caused by either the disease or, as often happens, by the treatments.
Acknowledgements
Disclosure: The author owns shares in several nutrition and probiotic-marketing companies.
References
- Guggenbichler JP, Assadian O, Boeswald M, et al. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials - catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip 2011;6:Doc18. [PubMed]
- Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the twenty first century (2000-2007). Chest 2011;140:1223-31. [PubMed]
- Hall MJ, Williams SJ, DeFrances CJ, et al. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals 2012. Available online:
- Vincent JL. Increasing awareness of sepsis: World Sepsis Day. Increasing awareness of sepsis: World Sepsis Day. Crit Care 2012;16:152. [PubMed]
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139-44. [PubMed]
- Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012;380:1059-65. [PubMed]
- Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse eff ect of postoperative complications. Ann Surg 2005;242:326-41. [PubMed]
- Pearse RM, Harrison DA, James P, et al. Identifi cation and characterisation of the high-risk surgical population in the United Kingdom. Crit Care 2006;10:R81. [PubMed]
- Jhanji S, Thomas B, Ely A, et al. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia 2008;63:695-700. [PubMed]
- Findley G, Goodwin A, Protopappa K, et al. Knowing the risk: a review of the peri-operative care of surgical patients. London: National Confidential Enquiry into Patient Outcome and Death, 2011.
- Head J, Ferrie JE, Alexanderson K, et al. Diagnosis-specific sickness absence as a predictor of mortality: the Whitehall II prospective cohort study. BMJ 2008;337:a1469. [PubMed]
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418-28. [PubMed]
- Derogar M, Orsini N, Sadr-Azodi O, et al. Infl uence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615-9. [PubMed]
- Elias AC, Matsuo T, Grion CM, et al. Incidence and risk factors for sepsis in surgical patients: a cohort study. J Crit Care 2012;27:159-66. [PubMed]
- Beghetto MG, Victorino J, Teixeira L, et al. Parenteral nutrition as a risk factor or central venous catheter-related infection. JPEN J Parenter Enteral Nutr 2005;29:367-73. [PubMed]
- Wren SM, Ahmed N, Jamal A, et al. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg 2005;140:752-6. [PubMed]
- Bucher P, Gervaz P, Soravia C, et al. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg 2005;92:409-14. [PubMed]
- Bucher P, Gervaz P, Egger JF, et al. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Dis Colon Rectum 2006;49:109-12. [PubMed]
- Wunsch H, Linde-Zwirble WT, Angus DC, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010;38:1947-53. [PubMed]
- Haskel Y, Xu D, Lu Q, et al. Elemental diet-induced bacterial translocation can be hormonally modulated. Ann Surg 1993;217:634-42. [PubMed]
- Deitch EA, Xu D, Naruhn MB, et al. Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg 1995;221:299-307. [PubMed]
- Lange H. Multiorgan dysfunction syndrome: how water might contribute to its progression. J Cell Mol Med 2002;6:653-60. [PubMed]
- Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002;359:1812-8. [PubMed]
- Macafee DAL, Allison SP, Lobo DN. Some interactions between gastrointestinal function and fluid and electrolyte homeostasis. Curr Opin Clin Nutr Metab Care 2005;8:197-203. [PubMed]
- Wan JM, Teo TC, Babayan VK, et al. Invited comment: lipids and the development of immune dysfunction and infection. JPEN J Parenter Enteral Nutr 1988;12:43S-52S. [PubMed]
- van der Poll T, Coyle SM, Levi M, et al. Fat emulsion infusion potentiates coagulation activation during human endotoxemia. Thromb Haemost 1996;75:83-6. [PubMed]
- Lin BF, Huang CC, Chiang BL, et al. Dietary fat influences Ia antigen expression, cytokines annd prostaglandin E2 production in immune cells in autoimmune-prone NZBxNZW F1 mice. Br J Nutr 1996;75:711-22. [PubMed]
- Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978-82. [PubMed]
- Mesotten D, Van den Berghe G. Clinical potential of insulin therapy in critically ill patients. Drugs 2003;63:625-36. [PubMed]
- Mesotten D, Van den Berghe G. Glycemic targets and approaches to management of the patient with critical illness. Curr Diab Rep 2012;12:101-7. [PubMed]
- Bulger EM. 7.5% saline and 7.5% saline/6% dextran for hypovolemic shock. J Trauma 2011;70:S27-9. [PubMed]
- Elmér O, Göransson G, Saku M, et al. Influence of physiological saline, dextran 70, hydroxyethyl starch, degraded gelatin, and fat emulsion solutions on screen filtration pressure. Eur Surg Res 1977;9:85-95. [PubMed]
- Zoucas E, Göransson G, Bengmark S. Colloid-induced changes in bleeding following liver resection in the rat. Res Exp Med (Berl) 1984;184:251-8. [PubMed]
- Andersson R, Alwmark A, Bengmark S. Influence of dextran on pneumococcal septicemia in splenic artery-ligated or splenectomized rats. Res Exp Med (Berl) 1987;187:423-7. [PubMed]
- Andersson R, Tranberg KG, Alwmark A, et al. Factors influencing the outcome of E. coli peritonitis in rats. Acta Chir Scand 1989;155:155-7. [PubMed]
- Alderson P, Schierhout G, Roberts I, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2000;2:CD000567. [PubMed]
- Reinhart K, Perner A, Sprung CL, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med 2012;38:368-83. [PubMed]
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl Starch 130/0.42 versus Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N Engl J Med 2012;367:124-34. [PubMed]
- Sandström R, Drott C, Hyltander A, et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in a randomized study. Ann Surg 1993;217:185-95. [PubMed]
- Charlson ME, MacKenzie CR, Gold JP, et al. Intraoperative blood pressure. What patterns identify patients at risk for postoperative complications? Ann Surg 1990;212:567-80. [PubMed]
- Haskel Y, Xu D, Lu Q, et al. Bombesin protects against bacterial translocation induced by three commercially available liquid enteral diets: a prospective, randomized, multigroup trial. Crit Care Med 1994;22:108-13. [PubMed]
- Haskel Y, Xu D, Lu Q, et al. The modulatory role of gut hormones in elemental diet and intravenous total parenteral nutrition-induced bacterial translocation in rats. JPEN - J Parenter Enteral Nutr 1994;18:159-66. [PubMed]
- Slotwinski R, Olszewski WL, Slotkowski M, et al. Can the interleukin-1 receptor antagonist (IL-1ra) be a marker of anti-inflammatory response to enteral immunonutrition in malnourished patients after pancreaticoduodenectomy? JOP 2007;8:759-69. [PubMed]
- Oz HS, Chen TS, Neuman M. Nutrition intervention: a strategy against systemic inflammatory syndrome. JPEN J Parenter Enteral Nutr 2009;33:380-9. [PubMed]
- Kaido T, Ogura Y, Ogawa K, et al. Effects of post-transplant enteral nutrition with an immunomodulating diet containing hydrolyzed whey peptide after liver transplantation. World J Surg 2012;36:1666-71. [PubMed]
- Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma. Crit Care Med 1999;27:733-40. [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14. [PubMed]
- Kalff JC, Carlos TM, Schraut WH, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 1999;117:378-87. [PubMed]
- de Jonge WJ, van den Wijngaard RM, The FO, et al. Postoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in mice. Gastroenterology 2003;125:1137-47. [PubMed]
- Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology 1992;103:807-12. [PubMed]
- Wei L, Wei H, Frenkel K. Sensitivity to tumor promotion of SENCAR and C57BL/6J mice correlates with oxidative events and DNA damage. Carcinogenesis 1993;14:841-7. [PubMed]
- Steinberg KP, Milberg JA, Martin TA, et al. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;150:113-22. [PubMed]
- Sookhai S, Wang JJ, McCourt M, et al. A novel therapeutic strategy for attenuating neutrophil-mediated lung injury in vivo. Ann Surg 2002;235:285-91. [PubMed]
- Ho JS, Buchweitz JP, Roth RA, et al. Identification of factors from rat neutrophils responsible for cytotoxicity to isolated hepatocytes. J Leukoc Biol 1996;59:716-24. [PubMed]
- Lowell CA, Bertin G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. PNAS - Proc Natl Acad Sci USA 1998;95:7580-4. [PubMed]
- Goris RJ, Boekholtz WK, van Bebber IP, et al. Multiple-organ failure and sepsis without bacteria. An experimental model. Arch Surg 1986;121:897-901. [PubMed]
- Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol 2005;288:L599-L607. [PubMed]
- Rassias AJ, Marrin CA, Arruda J, et al. Insulin infusion improves neutrophil function in diabetic cardiac surgery patients. Anesth Analg 1999;88:1011-6. [PubMed]
- O’Brien G, Shields CJ, Winter DC, et al. Cyclooxygenase-2 plays a central role in the genesis of pancreatitis and associated lung injury. Hepatobiliary Pancreat Dis Int 2005;4:126-9. [PubMed]
- Bengmark S. Control of systemic inflammation and chronic disease - the use of turmeric and curcumenoids. In: Mine Y, Miyashita K, Shahidi F. Nutrigenomics and proteonomics in health and disease. Food factors and gene interaction. Chichester, West Sussex, UK: Wiley-Blackwell, 2009:161-80.
- Lee JC, Kinniry PA, Arguiri E, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res 2010;173:590-601. [PubMed]
- Landi-Librandi AP, Caleiro Seixas Azzolini AE, de Oliveira CA, et al. Inhibitory activity of liposomal flavonoids during oxidative metabolism of human neutrophils upon stimulation with immune complexes and phorbol ester. Drug Deliv 2012;19:177-87. [PubMed]
- Ilkgul O, Aydede H, Erhan Y, et al. Subcutaneous administration of live lactobacillus prevents sepsis-induced lung organ failure in rats. Br J Int Care 2005;15:52-7.
- Tok D, Ilkgul O, Bengmark S, et al. Pretreatment with pro- and synbiotics reduces peritonitis-induced acute lung injury in rats. J Trauma 2007;62:880-5. [PubMed]
- Shimizu K, Ogura H, Goto M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma 2006;60:126-33. [PubMed]
- Shimizu K, Ogura H, Asahara T, et al. Gastrointestinal dysmotility is associated with altered gut flora and septic mortality in patients with severe systemic inflammatory response syndrome: a preliminary study. Neurogastroenterol Motil 2011;23:330-5. [PubMed]
- Shimizu K, Ogura H, Goto M, et al. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig Dis Sci 2009;54:1071-8. [PubMed]
- Ekberg H. Colorectal liver cancer, resection and regional chemotherapy. Bulletin from Department of Surgery. Lund University 1986;61:1-76.
- Ekberg H, Tranberg KG, Andersson R, et al. Major liver resection: perioperative course and management. Surgery 1986;100:1-8. [PubMed]
- Gustafsson BE. The physiological importance of the colonic microflora. Scand J Gastroenterol Suppl 1982;77:117-31. [PubMed]
- Gilliland SE, Speck ML. Antagonistic action of Lactobacillus acidophilus towards intestinal and food-borne pathogens in associative cultures. J Food Prot 1977;40:820-23.
- Molin G, Andersson R, Ahrné S, et al. Effect of fermented oatmeal soup on the cholesterol level and the Lactobacillus colonization of rat intestinal mucosa. Antonie Van Leeuwenhoek 1992;61:167-73. [PubMed]
- Molin G, Jeppsson B, Johansson ML, et al. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J Appl Bacteriol 1993;74:314-23. [PubMed]
- Johansson ML, Molin G, Jeppsson B, et al. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environ Microbiol 1993;59:15-20. [PubMed]
- Ljungh Å, Lan JG, Yamagisawa N. Isolation, selection and characteristics of Lactobacillus paracasei ssp paracasei isolate F19. Microb EcolHealth Dis 2002;4-6.
- Kruszewska K, Lan J, Lorca G, et al. Selection of lactic acid bacteria as probiotic strains by in vitro tests. Microecol Ther 2002;29:37-51.
- Rayes N, Hansen S, Seehöfer D, et al. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition 2002;18:609-15. [PubMed]
- Rayes N, Seehöfer D, Theruvath T, et al. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg 2007;246:36-41. [PubMed]
- Rayes N, Seehöfer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002;74:123-7. [PubMed]
- Rayes N, Seehöfer D, Theruvath T, et al. Combined perioperative enteral supply of bioactive pre- and probiotics abolishes postoperative bacterial infections in human liver transplantation - a randomised, double blind clinical trial. Am J Transplant 2005;5:125-30. [PubMed]
- Spindler-Vesel A, Bengmark S, Vovk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr 2007;31:119-26. [PubMed]
- Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, et al. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg 2006;30:1848-55. [PubMed]
- Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, et al. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma 2009;67:815-21. [PubMed]
- Koutelidakis IM, Bezirtzoglou E, Giamarellos-Bourboulis EJ, et al. Impact of synbiotics on the intestinal flora of critically ill patients with multiple injuries. Int J Antimicrob Agents 2010;36:90-1. [PubMed]
- Oláh A, Belágyi T, Issekutz Á, et al. Early enteral nutrition with specific lactobacillus and fibre reduces sepsis in severe acute pancreatitis. Brit J Surg 2002;89:1103-7. [PubMed]
- Oláh A, Belágyi T, Pótó L, et al. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology 2007;54:590-4. [PubMed]
- Plaudis H, Pupelis G, Zeiza K, et al. Early Low Volume Oral Synbiotic/Prebiotic Supplemented Enteral Stimulation of the Gut in Patients with Severe Acute Pancreatitis: A Prospective Feasibility Study. Acta Chir Belg 2012;112:131-8. [PubMed]
- Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 2011;53:1372-6. [PubMed]
- Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut 2007;56:1495-7. [PubMed]
- Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012;37:1885-95. [PubMed]
- Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441-9. [PubMed]
- Riordan SM, Skinner NA, McIver CJ, et al. Synbiotic-associated improvement in liver function in cirrhotic patients: Relation to changes in circulating cytokine messenger RNA and protein levels. Microb Ecol Health Dis 2007;19:7-16.
- Bengmark S. Bio-ecological control of chronic liver disease and encephalopathy. Metab Brain Dis 2009;24:223-36. [PubMed]
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365-71. [PubMed]
- Gori A, Tincati C, Rizzardini G, et al. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol 2008;46:757. [PubMed]
- Ellis CL, Ma ZM, Mann SK, et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr 2011;57:363-70. [PubMed]
- Cunningham-Rundles S, Ahrné S, Johann-Liang R, et al. Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection. Nutrients 2011;3:1042-70. [PubMed]
- Hummelen R, Changalucha J, Butamanya NL, et al. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. Int J Gynaecol Obstet 2010;111:245-8. [PubMed]
- Hummelen R, Changalucha J, Butamanya NL, et al. Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microbes 2011;2:80-5. [PubMed]
- Hummelen R, Hemsworth J, Changalucha J, et al. Effect of micronutrient and probiotic fortified yogurt on immune-function of anti-retroviral therapy naive HIV patients. Nutrients 2011;3:897-909. [PubMed]
- Schunter M, Chu H, Hayes TL, et al. Randomized pilot trial of a synbiotic dietary supplement in chronic HIV-1 infection. BMC Complement Altern Med 2012;12:84. [PubMed]
- Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nature 2005;437:1032-7. [PubMed]
- Loza MJ, McCall CE, Li L, et al. Assembly of inflammation-related genes for pathway-focused genetic analysis. PLoS One 2007;2:e1035. [PubMed]
- Seok J, Xiao W, Moldawer LL, et al. A dynamic network of transcription in LPS-treated human subjects. BMC Syst Biol 2009;3:78. [PubMed]
- Burcelin R, Serino M, Chabo C, et al. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol 2011;48:257-73. [PubMed]
- Festi D, Schiumerini R, Birtolo C, et al. Gut microbiota and its pathophysiology in disease paradigms. Dig Dis 2011;29:518-24. [PubMed]
- Leavy O. Inflammation: Trauma kicks up a storm. Nat Rev Immunol 2011;12:3. [PubMed]
- London NR, Zhu W, Bozza FA, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2010;2:23ra19. [PubMed]
- Müller-Ladner U, Pap T, Gay RE, et al. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol 2005;1:102-10. [PubMed]
- Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis NF-jB and its relevance to arthritis and inflammation. Rheumatology 2008;47:584-90. [PubMed]
- Perrier C, Rutgeerts P. Cytokine blockade in inflammatory bowel diseases. Immunotherapy 2011;3:1341-52. [PubMed]
- Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol 2011;27:635-62. [PubMed]
- Aggarwal BB, Sethi G, Baladandayuthapani V, et al. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem 2007;102:580-92. [PubMed]
- Bengmark S. Gut microbiota, immune development and function. Pharmacol Res 2012. pii: S1043-6618(12)00166-1.
- Bengmark S. Advanced glycation and lipoxidation end products--amplifiers of inflammation: the role of food. JPEN J Parenter Enteral Nutr 2007;31:430-40. [PubMed]
- Bengmark S. AGE, ALE RAGE and disease - a foods perspective. In: Susan S Cho. Handbook of Prebiotic and Probiotic Ingredients: Health Benefits and Food Applications. Boca Raton, Terry Finocchiaro CRC Press, Taylor and Francis Group, 2010:139-162.
- Bengmark S. Modified Amino Acid-Based Molecules: Accumulation and Health Implications. In: Ed Mello JFD. Amino Acids in Human Nutrition and Health. UK, CABI Wallingford, 2011:382-405.
- Alvarez-Jubete L, Arendt EK, Gallagher E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int J Food Sci Nutr 2009;60:240-57. [PubMed]
- Vanschoonbeek K, Lansink M, van Laere KM, et al. Slowly digestible carbohydrate sources can be used to attenuate the postprandial glycemic response to the ingestion of diabetes-specific enteral formulas. Diabetes Educ 2009;35:631-40. [PubMed]
- Martindale RG, DeLegge M, McClave S, et al. Nutrition delivery for obese ICU patients: delivery issues, lack of guidelines, and missed opportunities. JPEN J Parenter Enteral Nutr 2011;35:80S-7S. [PubMed]
- Miner-Williams W, Deglaire A, Benamouzig R, et al. Endogenous proteins in terminal ileal digesta of adult subjects fed a casein-based diet. Am J Clin Nutr 2012;96:508-15. [PubMed]
- Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, et al. A breakfast with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin Nutr 2009;28:147-55. [PubMed]
- Ang M, Müller AS, Wagenlehner F, et al. Combining protein and carbohydrate increases postprandial insulin levels but does not improve glucose response in patients with type 2 diabetes. Metabolism 2012;61:1696-702. [PubMed]
- Liu X, Murali SG, Holst JJ, et al. Whey protein potentiates the intestinotrophic action of glucagon-like peptide-2 in parenterally fed rats. Am J Physiol Regul Integr Comp Physiol 2009;297:R1554-62. [PubMed]
- Tang JE, Moore DR, Kujbida GW, et al. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987-92. [PubMed]
- Morifuji M, Sakai K, Sugiura K. Dietary whey protein modulates liver glycogen level and glycoregulatory enzyme activities in exercise-trained rats. Exp Biol Med (Maywood) 2005;230:23-30. [PubMed]
- Ebaid H, Salem A, Sayed A, et al. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis 2011;10:235. [PubMed]
- Hoppe C, Mølgaard C, Dalum C, et al. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr 2009;63:1076-83. [PubMed]
- Graf S, Egert S, Heer M. Effects of whey protein supplements on metabolism: evidence from human intervention studies. Curr Opin Clin Nutr Metab Care 2011;14:569-80. [PubMed]
- Yang L, Chen JH, Xu T, et al. Rice protein improves oxidative stress by regulating glutathione metabolism and attenuating oxidative damage to lipids and proteins in rats. Life Sci 2012;91:389-94. [PubMed]
- Chintagari NR, Liu L. GABA receptor ameliorates ventilator-induced lung injury in rats by improving alveolar fluid clearance. Crit Care 2012;16:R55. [PubMed]
- Schep LJ, Knudsen K, Slaughter RJ, et al. The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol. Clin Toxicol (Phila) 2012;50:458-70. [PubMed]
- Coda R, Rizzello CG, Gobbetti M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of gamma-aminobutyric acid (GABA). Int J Food Microbiol 2010;137:236-45. [PubMed]
- Coda R, Di Cagno R, Rizzello CG, et al. Utilization of African grains for sourdough bread making. J Food Sci 2011;76:M329-35. [PubMed]
- Han YY, Lai SL, Ko WJ, et al. Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract 2012;27:91-8. [PubMed]
- Versleijen MW, Roelofs HM, Rombouts C, et al. Short-term infusion of a fish oil-based lipid emulsion modulates fatty acid status, but not immune function or (anti)oxidant balance: a randomized cross-over study. Eur J Clin Invest 2012;42:290-302. [PubMed]
- Levy BD. Resolvins and protectins: natural pharmacophores for resolution biology. Prostaglandins Leukot Essent Fatty Acids 2010;82:327-32. [PubMed]
- Ott J, Hiesgen C, Mayer K. Lipids in critical care medicine. Prostaglandins Leukot Essent Fatty Acids 2011;85:267-73. [PubMed]
- van der Meij BS. n-3 PUFAs in cancer, surgery, and critical care: a systematic review on clinical effects, incorporation, and washout of oral or enteral compared with parenteral supplementation. Am J Clin Nutr 2011;94:1248-65. [PubMed]
- Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011;306:1574-81. [PubMed]
- Wang J, Yu JC, Kang WM, et al. Superiority of a fish oil-enriched emulsion to medium-chain triacylglycerols/long-chain triacylglycerols in gastrointestinal surgery patients: a randomized clinical trial. Nutrition 2012;28:623-9. [PubMed]
- Umpierrez GE, Spiegelman R, Zhao V, et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med 2012;40:1792-8. [PubMed]
- Siqueira J, Smiley D, Newton C, et al. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab 2011;96:3207-16. [PubMed]
- Unlu NZ, Bohn T, Clinton SK, et al. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr 2005;135:431-6. [PubMed]
- Boileau C, Martel-Pelletier J, Caron J, et al. Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res Ther 2009;11:R41. [PubMed]
- Oguntibeju OO, Esterhuyse AJ, Truter EJ. Red palm oil: nutritional, physiological and therapeutic roles in improving human wellbeing and quality of life. Br J Biomed Sci 2009;66:216-22. [PubMed]
- Sen CK, Rink C, Khanna S. Palm oil-derived natural vitamin E alpha-tocotrienol in brain health and disease. J Am Coll Nutr 2010;29:314S-323S. [PubMed]
- Takeuchi H, Sekine S, Kojima K, et al. The application of medium-chain fatty acids: edible oil with a suppressing effect on body fat accumulation. Asia Pac J Clin Nutr 2008;17:320-3. [PubMed]
- Wu SJ, Liu PL, Ng LT. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol Nutr Food Res 2008;52:921-9. [PubMed]
- Esterhuyse AJ, Toit ED, Rooyen JV. Dietary red palm oil supplementation protects against the consequences of global ischemia in the isolated perfused rat heart. Asia Pac J Clin Nutr 2005;14:340-7. [PubMed]
- Esterhuyse AJ, du Toit EF, Benadè AJ, et al. Dietary red palm oil improves reperfusion cardiac function in the isolated perfused rat heart of animals fed a high cholesterol diet. Prostaglandins Leukot Essent Fatty Acids 2005;72:153-61. [PubMed]
- van Rooyen J, Esterhuyse AJ, Engelbrecht AM, et al. Health benefits of a natural carotenoid rich oil: a proposed mechanism of protection against ischaemia/ reperfusion injury. Asia Pac J Clin Nutr 2008;17:316-9. [PubMed]
- Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 2010;85:1629-42. [PubMed]
- Thomas EL, Saeed N, Hajnal JV, et al. Magnetic resonance imaging of total body fat. J Appl Physiol 1998;85:1778-85. [PubMed]
- Alessi MC, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 1997;46:860-7. [PubMed]
- Almersjö O, Bengmark S, Engevik L, et al. Serum lipids after extensive liver resection in man. Acta Hepatosplenol 1968;15:1-12. [PubMed]
- Bengmark S. Liver steatosis and liver resection. Digestion 1969;2:304-11. [PubMed]
- Compare D, Coccoli P, Rocco A, et al. Gut--liver axis: the impact of gut microbiota on non alcoholicfatty liver disease. Nutr Metab Cardiovasc Dis 2012;22:471-6. [PubMed]
- Bengmark S, Olsson R. Effect of vitamin B12 on liver regeneration after partial hepatectomy. Gastroenterologia 1963;100:75-86. [PubMed]
- Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother 2012;3:149-55. [PubMed]
- Bengmark, S. Synbiotics in human Medicine. In: Versalovic J, Wilson M. Therapeutic Microbiology: Probiotics and Related Strategies. Washington DC USA, ASM Press, 2008:307-321.
- Xu RY, Wan YP, Fang QY, et al. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr 2012;50:72-7. [PubMed]
- Seehofer D, Schirmeier A, Bengmark S, et al. Curcumin attenuates oxidative stress and inflammatory response in the early phase after partial hepatectomy with simultaneous intraabdominal infection in rats. J Surg Res 2010;159:497-502. [PubMed]
- Kuo JJ, Chang HH, Tsai TH, et al. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis. Int J Mol Med 2012;30:673-9. [PubMed]
- Descotes J. Immunotoxicology: role in the safety assessment of drugs. Drug Saf 2005;28:127-36. [PubMed]
- Boutenko V. Green for life. Berkeley, CA, USA, North Atlantic Books, 2010.
- Shimizu K, Ogura H, Asahara T, et al. Probiotic/Synbiotic Therapy for Treating Critically Ill Patients from a Gut Microbiota Perspective. Dig Dis Sci 2013;58:23-32. [PubMed]
- Iapichino G, Callegari ML, Marzorati S, et al. Impact of antibiotics on the gut microbiota of critically ill patients. J Med Microbiol 2008;57:1007-14. [PubMed]