
Intention-to-treat approach for survival benefit of ABO-incompatible living-donor liver transplantation in patients with high Model for End-stage Liver Disease scores
Highlight box
Key findings
• Intention-to-treat analysis revealed that ABO-incompatible (ABOi) living-donor liver transplantation (LDLT) significantly improved survival rates for patients with Model for End-stage Liver Disease (MELD) ≥30 with reasonable posttransplant outcomes.
What is known and what is new?
• ABOi-LDLT is a viable option in organ shortage region; however, its applicability for patients with high MELD scores remains uncertain.
• Among high MELD score waitlisted population, those with the intention of ABOi-LDLT demonstrated improved survival rates over awaiting deceased-donor liver transplantation (DDLT). The posttransplant outcomes of ABOi-LDLT recipients were comparable to those with DDLT and even ABO-compatible LDLT.
What is the implication, and what should change now?
• ABOi-LDLT may be considered in high MELD patients when patients only have eligible living-donor with ABO-mismatch.
Introduction
Living-donor liver transplantation (LDLT) is a viable treatment option for patients with end-stage liver disease or hepatocellular carcinoma (1-5). However, while it is crucial to minimize risks to the donor and maximize benefits for the recipient, finding a suitable living donor presents a significant challenge (6). ABO-incompatible (ABOi)-LDLT serves as a treatment plan to expand the pool of potential living donors (7,8). The use of ABOi is increasing significantly, particularly in Asian countries with severe organ shortages (9).
Despite advances in desensitization protocols, including anti-CD20 monoclonal antibody (rituximab) and therapeutic plasma exchange (TPE) improved outcomes of ABOi-LDLT (10), ABOi desensitization still presents its own set of complications. Patients generally require a desensitization period that makes them more susceptible to infection and bleeding (11,12). Biliary complications also remain more prevalent due to antibody-mediated rejection that results in intrahepatic necrosis, chronic biliary damage (13,14), and subsequent decreased graft survival rates (15). However, the benefits and risks of ABOi-LDLT remain to be determined, and recent study findings suggest it offers comparable post-transplant outcomes (16-18).
In situations of severe organ shortage, patients with high Model for End-stage Liver Disease (MELD) scores have a greater chance of receiving a deceased-donor liver transplantation (DDLT) allocation (19,20). However, they also face a greater risk of mortality during the waiting period, because only few patients with extremely high MELD scores could receive DDLT (21-23). In these patients, LDLT could be a favorable treatment choice because it enables prompt and timely intervention. In our previous research, we highlighted the advantages of LDLT in patients with a MELD score ≥30, compared with waiting for DDLT in regions with severe organ shortage. In South Korea, the rates of DDLT between 2021 and 2020 was only 24.7–32.6%. Specifically, the proportion of patients with MELD scores of 39 or higher allocated to DDLT in 2021 was overwhelmingly high (23). Nonetheless, there are limited reports regarding the viability of ABOi-LDLT in patients with high MELD scores. Given the increased risk of complications and rejection associated with ABOi-LDLT, the feasibility of ABOi-LDLT in patients with high MELD scores may vary depending on the condition of the candidates. To our knowledge, the Lee et al. study is the only investigation in 5 years. They found a 75% 1-year survival rate for eight recipients who underwent ABOi-LDLT due to acute liver failure; the median MELD score in this group was 39. However, this study was not designed as a comparative analysis and has limitations in terms of effectively demonstrating the survival benefit of ABOi-LDLT (24).
This study used an intention-to-treat (ITT) approach to investigate the survival benefit of ABOi-LDLT in patients who were waitlisted with a MELD score ≥30. We also aimed to compare the post-transplant outcomes of ABOi-LDLT with those of DDLT and ABO-compatible (ABOc)-LDLT. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-58/rc).
Methods
Study population
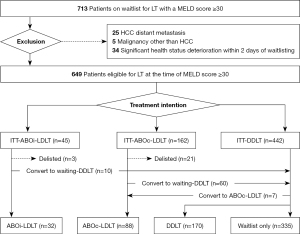
Records for 713 patients with MELD scores ≥30 and who were on the waitlist for liver transplantation (LT) at Severance Hospital were reviewed for the September 2005 to December 2021 period. Sixty-four patients with hepatocellular carcinoma with distant metastasis, any other malignancy diagnosed within 5 years, or significant deterioration in health status within 2 days of placement on the waitlist were excluded during the review.
The 649 patients included in this study were divided into three groups based on treatment intention and potential donor type at the beginning of the evaluation, irrespective of actual treatment received: those who were to receive ABOi-LDLT (“ITT-ABOi-LDLT group”) or ABOc-LDLT (“ITT-ABOc-LDLT group”), or who were to wait for DDLT (“ITT-DDLT group”). During the final analysis, we further classified patients into four groups based on actual treatment method: those who underwent ABOi-LDLT, ABOc-LDLT, DDLT, or who remained on the waitlist. Patients who had potential donors were considered to have an intention for LDLT because they underwent series of evaluations, described in the “Enlistment for transplantation and living-donor evaluation” section. If patient who were planned to undergo LDLT experienced an acute deterioration in their health condition that rendered them unable to undergo the transplantation, they were subsequently delisted.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Severance Hospital approved the study (No. 4-2022-0913), and patient consent for this study was waived because of its retrospective design.
Enlistment for transplantation and living-donor evaluation
All patients requiring LT are initially registered for DDLT. During the evaluation process, their medical conditions are thoroughly reviewed, including the presence of active infection, thrombosis or obliteration in the portal vein, esophageal/gastric varices, other complications related to end-stage liver disease and hidden malignancies. Concurrently, patients are inquired about the availability of a potential living-donor with clear willingness among their family members (Appendix 1). If such a potential donor is identified, the evaluation process for LDLT begins.
For the potential donors, a series of evaluations were performed to investigate the health condition, their ABO match with patient, anatomy of the liver. Suitable graft is selected as described previously (25). When ABOi-LDLT was planned, immunoglobulin M (IgM) and immunoglobulin G (IgG) of anti-A/B results were also examined for potential recipient.
ABO desensitization protocol
We previously documented our institutional protocol of desensitization for ABOi-LDLT based on rituximab and TPE (26), which was revised once (Appendix 2). We used the higher values for IgM or IgG titers of anti-A/B as the initial or target isoagglutinin titers. The number of rounds (limited to six) of preoperative TPE was determined based on initial isoagglutinin titer. Splenectomy and postoperative TPE were performed when patients had a high risk of rejection, such as an isoagglutinin titer of LT >1:64. We performed additional rounds of TPE postoperatively when patients had features of clinical rejection or isoagglutinin titer rebound, which was defined as both a resurgence to 1:64 of the isoagglutinin titer and a minimum two-fold increase. After TPE, intravenous immunoglobulin was used on a patient-by-patient basis at a dose of 500–800 mg/kg, according to ABO antibody strength and risk of infection.
Variables and definitions
Age, sex, ABO type, underlying liver disease, and hepatocellular carcinoma progression exceeding Milan criteria were recorded for each patient. An uncapped MELD score was also calculated (27). Acute liver failure (28,29), acute-on-chronic liver failure graded 0 to 3, and chronic liver failure were identified (30). Chronic kidney disease was defined as a diagnosis within 3 months before a MELD score of 30 and an estimated glomerular filtration rate <60 mL/min/1.73 m2. Patients without chronic kidney disease who had serum creatinine levels ≥1.5 mg/dL were classified as having hepatorenal syndrome (31). Cerebrovascular disease included heart failure, coronary artery disease, valvular heart disease, cerebral hemorrhage, and cerebrovascular accident. Intensive care unit stays were documented pre-transplantation.
Patient survival according to treatment intention was calculated from the date of waitlisting until the date of patient death or December 31, 2021 (end of follow-up period). Post-transplant survival was calculated from the date of LT. Data on in-hospital death, graft loss within a year, postoperative hospital stay, re-transplantation rate, complications, and rejection were also collected. Graft loss was defined as death or re-transplantation due to graft failure. Major complications were defined as grade ≥3 complications using the Clavien-Dindo classification system (32).
Statistical analysis
Results were presented as numbers (percentages) for categorical variables. For continuous variables, the results were shown as either mean and standard deviation or median and interquartile range values. Additionally, to ensure detailed information, median values accompanied by their respective ranges were employed specifically in the descriptions related to ABO desensitization. To determine the significance of intergroup differences, Fisher’s exact test was used for categorical variables; the t-test or Mann-Whitney U test was used for continuous variables. The Kaplan-Meier method was used to analyze patient survival rates. The log-rank test and post-hoc analysis was performed to compare survival rates between groups. We used Multivariate logistic regression analysis to identify variables independently associated with survival in the treatment intention population and the post-transplant groups. Clinically important variables and variables with a P value ≤0.20 in univariate analyses were introduced into multivariate regression models.
Propensity scoring was used to compare survival after matching between the ITT-ABOi-LDLT and the other two groups. Propensity scores were calculated with variables selected from binomial logistic regression analysis that included all baseline variables. The variables were matched using a ratio of 1:2, the nearest neighbor matching algorithm without replacement; distances were determined using logistic regression analysis. The balance between groups was considered appropriate if the standardized mean differences were <0.20. Individuals were discarded from both matching groups if there was no appropriate match during between-group comparisons.
All calculations were performed using R 4.2.0 for MacOS (https://cran.r-project.org/; R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS 26 (SPSS Inc., Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
Study participants
A total of 649 patients were included in the study, with median duration of follow-up of 59 days. Among the included population, the ITT-ABOi-LDLT group consisted of 45 (6.9%) patients, the ITT-ABOc-LDLT group consisted of 162 (25.0%) patients, and the ITT-DDLT group consisted of 442 (68.1%) patients. Figure 1 presents a flowchart for the intended and actual treatments. Causes of withdrawal for patients who did not receive the treatment of initial intention are presented in Appendix 3. Unfortunately, five patients did not complete the desensitization due to not reaching the target titer despite desensitization (n=2), desensitization discontinued due to infection (n=2), or allocation to DDLT during desensitization (n=1) (Appendix 4). Thirty-two (4.9%) of 649 study participants received ABOi-LDLT, 88 (13.6%) received ABOc-LDLT, 170 (26.2%) received DDLT, and 335 (51.6%) patients remained on the waitlist.
Baseline characteristics on waitlisting day
Table 1 presents the results for baseline characteristics based on treatment intention. Most characteristics were similar between groups, including sex, underlying liver disease type, and MELD scores. However, there were some initial among-group differences. The ITT-ABOi-LDLT group had a younger median age than the ITT-DDLT group (52.0 vs. 54.0 years, respectively, P=0.35). The ITT-ABOi-LDLT group had lower grades of acute-on-chronic liver failure than both the ITT-DDLT (ACLF grade 0, 33.3% vs. 7.2%, respectively, P<0.001) and ITT-ABOc-LDLT (ACLF grade 0, 33.3% vs. 5.6%, respectively, P<0.001) groups. These patients also had a lower incidence of acute liver failure than the ITT-ABOc-LDLT group patients (6.7% vs. 17.9%, respectively, P<0.001). While ITT-ABOi-LDLT group patients exhibited a lower incidence of hyperbilirubinemia than the ITT-DDLT group (44.4% vs. 62.0%, respectively, P=0.03), they also had a higher incidence of coagulopathy compared with the ITT-DDLT group patients (68.9% vs. 48.6%, respectively, P=0.02) and were less likely to have hepatorenal syndrome than the ITT-DDLT group patients (35.6% vs. 56.3%, respectively, P=0.01). Moreover, ITT-ABOi-LDLT group patients had a higher likelihood of having hepatocellular carcinoma compared with the ITT-ABOc-LDLT group patients (33.3% vs. 12.3%, respectively, P=0.002); this group also had a greater proportion of patients whose MELD score increased rapidly within a month, compared with the ITT-ABOc-LDLT group (33.3% vs. 14.8%, respectively, P=0.003). Despite having a lower duration of intensive care unit stay compared with both the ITT-DDLT group (17.8 vs. 24.0 days, respectively, P=0.45) and ITT-ABOc-LDLT group (17.8 vs. 24.1 days, respectively, P=0.49), and a lower proportion of sepsis than the ITT-DDLT group (6.7% vs. 12.4%, respectively, P=0.37), no statistically significant difference was found.
Table 1
| Variables | ITT-ABOi-LDLT (n=45) | ITT-DDLT (n=442) | P† | ITT-ABOc-LDLT (n=162) | P‡ |
|---|---|---|---|---|---|
| Age (years) | 52.0 (44.0–61.0) | 54.0 (46.0–62.0) | 0.35 | 50.0 (43.0–56.0) | 0.45 |
| Male sex | 28 (62.2) | 319 (72.2) | 0.22 | 109 (67.3) | 0.65 |
| ABO type | 0.04 | 0.04 | |||
| A/B | 27 (60.0) | 257 (58.1) | 103 (63.6) | ||
| AB | 0 (0.0) | 52 (11.8) | 16 (9.9) | ||
| O | 18 (40.0) | 133 (30.1) | 43 (26.5) | ||
| Underlying liver disease | 0.35 | 0.19 | |||
| Alcoholic | 9 (20.0) | 108 (24.4) | 35 (21.6) | ||
| Hepatitis B | 18 (40.0) | 210 (47.5) | 73 (45.1) | ||
| Hepatitis C | 3 (6.7) | 25 (5.7) | 6 (3.7) | ||
| Hepatitis A | 0 (0.0) | 11 (2.5) | 10 (6.2) | ||
| Cryptogenic | 9 (20.0) | 54 (12.2) | 14 (8.6) | ||
| Autoimmune | 4 (8.9) | 16 (3.6) | 11 (6.8) | ||
| Others | 2 (4.4) | 18 (4.1) | 13 (8.0) | ||
| MELD score | 0.11 | 0.08 | |||
| 30–34 | 26 (57.8) | 309 (69.9) | 113 (69.8) | ||
| 35–39 | 15 (33.3) | 88 (19.9) | 29 (17.9) | ||
| ≥40 | 4 (8.9) | 45 (10.2) | 20 (12.3) | ||
| Liver failure types | <0.001 | <0.001 | |||
| ACLF 0§ | 15 (33.3) | 32 (7.2) | 9 (5.6) | ||
| ACLF 1§ | 2 (4.4) | 49 (11.1) | 11 (6.8) | ||
| ACLF 2§ | 14 (31.1) | 192 (43.4) | 72 (44.4) | ||
| ACLF 3§ | 11 (24.4) | 131 (29.6) | 41 (25.3) | ||
| Acute liver failure | 3 (6.7) | 38 (8.6) | 29 (17.9) | ||
| Organ failure§ | |||||
| Liver | 20 (44.4) | 274 (62.0) | 0.03 | 113 (69.8) | 0.003 |
| Brain | 3 (6.7) | 71 (16.1) | 0.15 | 21 (13.0) | 0.37 |
| Coagulation | 31 (68.9) | 215 (48.6) | 0.02 | 117 (72.2) | 0.80 |
| Circulation | 12 (26.7) | 107 (24.2) | 0.85 | 33 (20.4) | 0.48 |
| Respiratory | 5 (11.1) | 53 (12.0) | >0.99 | 18 (11.1) | 0.99 |
| Hepatorenal syndrome | 16 (35.6) | 249 (56.3) | 0.01 | 70 (43.2) | 0.45 |
| Chronic kidney disease | 2 (4.4) | 39 (8.8) | 0.47 | 2 (1.2) | 0.44 |
| Cardiovascular disease | 2 (4.4) | 29 (6.6) | 0.82 | 2 (1.2) | 0.44 |
| Intensive care unit stay (days) | 8 (17.8) | 106 (24.0) | 0.45 | 39 (24.1) | 0.49 |
| Renal replacement therapy | 5 (11.1) | 62 (14.0) | 0.75 | 16 (9.9) | 0.99 |
| Milan criteria | 0.89 | 0.002 | |||
| No HCC | 30 (66.7) | 290 (65.6) | 142 (87.7) | ||
| Within Milan | 10 (22.2) | 92 (20.8) | 16 (9.9) | ||
| Above Milan | 5 (11.1) | 60 (13.6) | 4 (2.5) | ||
| MELD score increases in a month | 0.10 | 0.003 | |||
| <15 | 10 (22.2) | 112 (25.3) | 23 (14.2) | ||
| ≥15 | 15 (33.3) | 87 (19.7) | 24 (14.8) | ||
| Present with ≥30 | 20 (44.4) | 243 (55.0) | 115 (71.0) | ||
| Sepsis | 3 (6.7) | 55 (12.4) | 0.37 | 11 (6.8) | 0.99 |
| Pneumonia | 4 (8.9) | 37 (8.4) | >0.99 | 12 (7.4) | 0.98 |
Data are presented as median (interquartile range) or n (%). †, P values, ITT-ABOi-LDLT vs. ITT-DDLT; ‡, P values, ITT-ABOi-LDLT vs. ITT-ABOc-LDLT; §, based on the reference (30). ITT, intention-to-treat; ABOi, ABO-incompatible; LDLT, living-donor liver transplantation; DDLT, deceased-donor liver transplantation; ABOc, ABO-compatible; MELD, Model for End-stage Liver Disease; ACLF, acute-on-chronic liver failure; HCC, hepatocellular carcinoma.
Patient survival after waitlisting, according to treatment intention
During follow-up, 14 deaths occurred in the ITT-ABOi-LDLT group, 302 in the ITT-DDLT group, and 76 in the ITT-ABOc-LDLT group. After waitlisting, the ITT-ABOi-LDLT group had a significantly higher 1-year patient survival after waitlisting than the ITT-DDLT group (66.7% vs. 28.7%, respectively, P<0.001, Figure 2) and ITT-ABOc-LDLT group (66.7% vs. 50.0%, P=0.04). A multivariate logistic regression analysis revealed that treatment intention of ABOi-LDLT was independently associated with a decreased risk of all-cause mortality after waitlisting [hazard ratio (HR), 0.32; P<0.001; Table 2]. Factors associated with an increased risk of all-cause mortality included older age, hepatocellular carcinoma, organ failure involving the brain, hepatorenal syndrome, cardiovascular disease, and AB blood type. Following propensity score matching, the ITT-ABOi-LDLT group still had a significantly greater 1-year patient survival after waitlisting than the ITT-DDLT group, but a similar 1-year patient survival after waitlisting than the ITT-ABOc-LDLT group (Appendices 5,6).
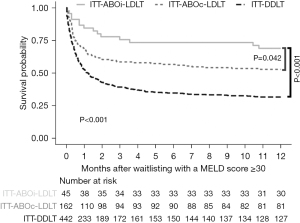
Table 2
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| ITT-DDLT | Reference | Reference | |||
| ITT-ABOi-LDLT | 0.31 (0.18–0.53) | <0.001 | 0.32 (0.18–0.55) | <0.001 | |
| ITT-ABOc-LDLT | 0.56 (0.44–0.72) | <0.001 | 0.75 (0.58–0.97) | 0.03 | |
| Age | 1.03 (1.02–1.04) | <0.001 | 1.03 (1.02–1.04) | <0.001 | |
| Male sex | 1.03 (0.83–1.28) | 0.78 | |||
| ABO type | |||||
| A/B | Reference | Reference | |||
| AB | 0.77 (0.54–1.11) | 0.17 | 0.67 (0.47–0.96) | 0.03 | |
| O | 1.17 (0.94–1.45) | 0.16 | 1.14 (0.92–1.42) | 0.23 | |
| Alcoholic liver disease | 0.74 (0.58–0.95) | 0.02 | |||
| MELD scores | |||||
| 30–34 | Reference | ||||
| 35–39 | 1.08 (0.84–1.39) | 0.53 | |||
| ≥40 | 1.04 (0.74–1.46) | 0.82 | |||
| Hepatocellular carcinoma | 2.32 (1.89–2.85) | <0.001 | 1.82 (1.46–2.27) | <0.001 | |
| Liver failure type | |||||
| ACLF 0† | Reference | ||||
| ACLF 1† | 2.12 (1.33–3.39) | 0.002 | |||
| ACLF 2† | 1.28 (0.85–1.93) | 0.23 | |||
| ACLF 3† | 2.30 (1.52–3.49) | <0.001 | |||
| Acute liver failure | 1.14 (0.69–1.89) | 0.61 | |||
| Organ failure† | |||||
| Brain | 2.15 (1.67–2.77) | <0.001 | 2.05 (1.59–2.65) | <0.001 | |
| Circulation | 1.52 (1.22–1.90) | <0.001 | |||
| Respiratory | 1.74 (1.31–2.32) | <0.001 | |||
| Hepatorenal syndrome | 1.52 (1.24–1.85) | <0.001 | 1.27 (1.04–1.56) | 0.02 | |
| Cardiovascular disease | 1.79 (1.21–2.64) | 0.004 | 1.59 (1.07–2.37) | 0.02 | |
| Intensive care unit stay (days) | 1.43 (1.14–1.79) | 0.002 | |||
| MELD score increases in a month | |||||
| <15 | Reference | ||||
| ≥15 | 1.44 (1.09–1.92) | 0.01 | |||
| Present with ≥30 | 0.74 (0.58–0.94) | 0.01 | |||
| Sepsis | 1.37 (1.02–1.85) | 0.03 | |||
| Pneumonia | 1.51 (1.08–2.10) | 0.02 | |||
†, based on the reference (30). HR, hazard ratio; CI, confidence interval; ITT, intention-to-treat; DDLT, deceased-donor liver transplantation; ABOi, ABO-incompatible; LDLT, living-donor liver transplantation; ABOc, ABO-compatible; MELD, Model for End-stage Liver Disease; ACLF, acute-on-chronic liver failure.
Characteristics at LT and postoperative outcomes
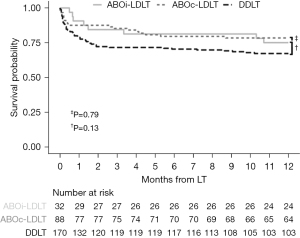
Among the patients who finally received LT, the ABOi-LDLT group was more likely to have lower MELD scores (Appendix 7). There were no differences in graft types or graft to recipient weight ratio between ABOi-LDLT and ABOc-LDLT groups. However, DDLT group exhibited a significant larger graft to recipient weight ratio, primarily due to the predominance of whole liver grafts. All three groups had similar waiting periods for LT. As presented in Figure 3, ABOi-LDLT group recipients had similar 1-year patient survival after LT compared to the DDLT (75% vs. 60.6%, respectively, P=0.13), and ABOc-LDLT (75% vs. 72.7%, respectively, P=0.79) group recipients. Multivariate logistic regression analysis found that ABOi-LDLT was not associated with graft loss, compared with the DDLT and ABOc-LDLT groups (Appendix 8). Regarding the causes of death, there was no significant difference among the three groups, as detailed in Appendix 9.
In-hospital mortality, graft loss, postoperative hospital stays, major complication rates, and rejection rates were similar between the three groups (Table 3). However, a detailed analysis found that ABOi-LDLT group recipients had an increased risk of biliary strictures, compared with DDLT group recipients (25% vs. 10%, respectively, P=0.04). In regard to the living donors, there was no mortality among the donors of either LDLT group. They also had similar postoperative hospital stays, readmission rates within 6 months, and major complication rates.
Table 3
| Complications | ABOi-LDLT (n=32) | DDLT (n=170) | P† | ABOc-LDLT (n=88) | P‡ |
|---|---|---|---|---|---|
| Recipient | |||||
| In-hospital mortality | 6 (18.8) | 46 (27.1) | 0.22 | 16 (18.2) | >0.99 |
| Graft loss within 1 year | 8 (25.0) | 55 (32.4) | 0.54 | 19 (21.6) | >0.99 |
| Postoperative hospital stay (days) | 24 [15–102] | 32 [0–1,224] | 0.09 | 26 [0–219] | >0.99 |
| Major complications (grade ≥3) | 0.21 | 0.97 | |||
| Biliary leakage | 4 (12.5) | 8 (4.7) | 0.19 | 10 (11.4) | >0.99 |
| Biliary stricture | 8 (25.0) | 17 (10.0) | 0.04 | 24 (27.3) | 0.29 |
| Hepatic artery complication | 3 (9.4) | 3 (1.8) | 0.08 | 2 (2.3) | 0.28 |
| Portal vein complication | 0 (0.0) | 4 (2.4) | 0.85 | 5 (5.7) | 0.19 |
| Hepatic vein or IVC complication | 1 (3.1) | 7 (4.1) | 0.99 | 1 (1.1) | >0.99 |
| Rejection within 1 year | 10 (31.3) | 33 (19.4) | 0.21 | 20 (22.7) | 0.48 |
| Biopsy proven rejection | 0 (0.0) | 10 (5.9) | 0.37 | 4 (4.5) | 0.32 |
| Antibody-mediated rejection | 0 (0.0) | 1 (0.6) | 0.99 | 0 (0.0) | – |
| Living donor | |||||
| Mortality | 0 (0.0) | 0 (0.0) | – | ||
| Postoperative hospital stay (days) | 8 [5–18] | 9 [5–31] | >0.99 | ||
| Readmission within 6 months | 1 (3.1)§ | 3 (3.4) | >0.99 | ||
| Major complications (grade ≥3) | >0.99 | ||||
| Biliary leakage | 0 (0.0) | 2 (2.3) | |||
| Biliary stricture | 1 (3.1) | 1 (1.1) | |||
| Intestinal obstruction | 0 (0.0) | 1 (1.1) |
Data are presented as n (%) or median [interquartile range]. †, P values, ABOi-LDLT vs. DDLT; ‡, P values, ABOi-LDLT vs. ABOc-LDLT; §, biliary stricture was the cause of readmission. ABOi, ABO-incompatible; LDLT, living-donor liver transplantation; DDLT, deceased-donor liver transplantation; ABOc, ABO-compatible; IVC, inferior vena cava.
Detailed information on recipient of ABOi-LDLT
All patients who underwent ABOi-LDLT received rituximab and more than one round of TPE for desensitization. Regarding use of preoperative immunosuppressants, 18 (56.3%) received tacrolimus, 8 (25.0%) were treated with mycophenolate mofetil, and 7 (21.9%) received intravenous immunoglobulin. All recipients were administered basiliximab as an induction immunosuppressant. Three recipients (9.4%) underwent splenectomy during the LT (Appendix 10).
When the 32 recipients who underwent ABOi-LDLT were reassigned according to initial isoagglutinin titer group, there were 12 (37.5%) with a titer ≤1:32, 11 (34.4%) with 1:64–1:128, 4 (12.5%) with 1:256–1:512, and 5 (15.6%) with 1:1,024–1:2,048 (Table 4). Recipients with a low initial isoagglutinin titer received a reduced dose of rituximab and a lower cycle of TPE. Recipients with a high initial isoagglutinin titer included those who underwent splenectomy. The results for postoperative outcomes indicated that in-hospital mortality was 18.8% (16.7% with ≤1:32, 9.1% with 1:64–1:128, 25.0% with 1:256–1:512, 40.0% with 1:1,024–1:2,048) and 25% had graft loss within a year (16.7%, 27.3%, 25.0%, 40.0%, consecutively). The median postoperative hospital stay was 24 days, with a range of 15–102 days (median 27, 22, 22, and 33 days, consecutively) and the rate of rejection within 1 year was 31.3% (41.7%, 36.4%, 0.0%, 20.0%, consecutively). Ten recipients (31.3%) had a major complication (50.0%, 18.2%, 25.0%, 20.0%, consecutively) and eight recipients (25.0%) had biliary stricture (41.7%, 18.2%, 25.0%, 0.0%, consecutively).
Table 4
| Variables | Initial IA-titer | ||||
|---|---|---|---|---|---|
| ~1:32 (n=12) | 1:64–1:128 (n=11) | 1:256–1:512 (n=4) | 1:1,024–1:2,048 (n=5) | Total (n=32) | |
| Desensitization | |||||
| Rituximab, 375 mg/m2 | 7 (58.3) | 9 (81.8) | 4 (100.0) | 5 (100.0) | 24 (75.0) |
| Preop. TPE, number | 2 [1–3] | 4 [2–7] | 4 [3–9] | 8 [6–11] | 3 [1–11] |
| Preop. IVIG | 0 (0.0) | 1 (9.1) | 2 (50.0) | 4 (80.0) | 7 (21.9) |
| Preop. MMF | 1 (8.3) | 2 (18.2) | 1 (25.0) | 1 (20.0) | 8 (25.0) |
| Preop. tacrolimus | 3 (25.0) | 4 (36.4) | 2 (50.0) | 4 (80.0) | 18 (56.3) |
| IA-titer at LT | 1:8 [1:1–1:32] | 1:8 [1:2–1:64] | 1:16 [1:4–1:64] | 1:32 [1:16–1:64] | 1:8 [1:1–1:64] |
| Splenectomy | 0 (0.0) | 0 (0.0) | 1 (25.0)† | 2 (40.0)‡ | 3 (9.4) |
| Postop. TPE | 2 (16.7) | 2 (18.2) | 0 (0.0) | 1 (20.0) | 5 (15.6) |
| Postop. IA-titer, max | 1:8 [1:4–1:512] | 1:16 [1:2–1:256] | 1:32 [1:32–1:64] | 1:32 [1:16–1:128] | 1:16 [1:2–1:512] |
| Post-transplant outcomes | |||||
| In-hospital mortality | 2 (16.7) | 1 (9.1) | 1 (25.0) | 2 (40.0) | 6 (18.8) |
| Graft loss within a year | 2 (16.7) | 3 (27.3) | 1 (25.0) | 2 (40.0) | 8 (25.0) |
| Postop. hospital stay (days) | 27 [18–102] | 22 [15–41] | 22 [15–38] | 33 [20–38] | 24 [15–102] |
| IA-titer rebound | 2 (16.7) | 2 (18.2) | 1 (25.0) | 1 (20.0) | 6 (18.8) |
| Rejection within a year | 5 (41.7) | 4 (36.4) | 0 (0.0) | 1 (20.0) | 10 (31.3) |
| Complications | 6 (50.0) | 2 (18.2) | 1 (25.0) | 1 (20.0) | 10 (31.3) |
| Biliary leakage | 3 (25.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 4 (12.5) |
| Biliary stricture | 5 (41.7) | 2 (18.2) | 1 (25.0) | 0 (0.0) | 8 (25.0) |
| Hepatic artery§ | 1 (8.3) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 2 (6.3) |
Data are presented as n (%) or median [range]. †, due to high IA-titer at LT (1:128). Male patient underwent additional TPE during the morning of the operation day, and the IA-titer decreased to 1:32; ‡, due to pancytopenia (one case), and high IA-titer at LT (another case, which was 1:64, for both IgM and IgG); §, complications were bleeding from pseudoaneurysm and occlusion. IA, isoagglutinin; preop., preoperative; TPE, therapeutic plasma exchange; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; LT, liver transplantation; postop., postoperative; IgM, immunoglobulin M; IgG, immunoglobulin G.
While the rate of isoagglutinin titer rebound was 18.8% among recipients in the ABOi-LDLT group (16.7%, 18.2%, 25.0%, and 20.0%, consecutively), there were no distinct characteristics for recipients who experienced isoagglutinin titer rebound (Appendix 11).
Discussion
ITT with ABOi-LDLT resulted in improved 1-year patient survival after waitlisting over awaiting DDLT as a waitlisted patient in regions with severe organ shortage; it was independently associated with reduced mortality in patients with a MELD score ≥30. The ITT-ABOi-LDLT group patients had a similar 1-year patient survival after waitlisting compared to the ITT-ABOc-LDLT group patients. Additional investigation comparing post-transplant outcomes among patients who underwent LT revealed that recipients of ABOi-LDLT had similar post-transplant outcomes to recipients of DDLT or ABOc-LDLT.
In regions where there is a severe shortage of organs from deceased donors, LDLT is a viable option for patients with high MELD scores, as it can mitigate waitlist-associated mortality through timely intervention (23,33). However, when a potential living donor has an ABO mismatch with the recipient, patients with elevated MELD scores require a period for pretransplant desensitization determined by the initial isoagglutinin titer value (11). This scenario could lead to a situation where patients might not complete desensitization because they need an urgent LT (34). In addition, increased risk of infection and bleeding due to administration of rituximab and TPE can become another barrier for patients with elevated MELD scores; this subpopulation of patients has a high prevalence of acute-on-chronic liver failure (35). Therefore, ABOi-LDLT has been performed on a limited basis in patients with high MELD scores. However, the use of ABOi-LDLT is becoming popular due to the increasing prevalence of non-alcoholic fatty liver disease (36-38) and the worsening organ shortage associated with a decrease in eligible donors (39). To our knowledge, this study was the first to investigate the feasibility of ABOi-LDLT in patients with high MELD scores with ITT analysis in a large sample size. Prior studies investigating outcomes of ABOi-LDLT included patients with low MELD scores or had small numbers of patients (Appendix 12). Therefore, this result provided evidence for use of ABOi-LDLT in patients with high MELD scores.
Patients in the ITT-ABOi-LDLT group had increased 1-year patient survival after waitlisting, compared with patients in the ITT-ABOc-LDLT group. Considering that propensity score matching eliminated the difference, patients in the ITT-ABOi-LDLT group may have had better conditions than the patients in the ITT-ABOc-LDLT group. They experienced a rapid increase in MELD scores 1 month before enlistment and were less likely to have a higher grade of acute-on-chronic liver failure and hyperbilirubinemia than the ITT-ABOc-LDLT group patients. Also, regarding the comparison between patients who received LT, the ABOi-LDLT group recipients had lower pretransplant MELD scores than the ABOc-LDLT group recipients. Undergoing TPE can contribute to deterioration of the coagulation system which results in a MELD score increase. However, studies indicate that TPE may have a survival benefit in patients with acute liver failure and acute-on-chronic liver failure (40,41). TPE can also provide the beneficial effect of rapid hemodynamic improvement during management of patients with sepsis (42). Although these results are limited, further investigations are needed to evaluate the benefits of TPE in patients with elevated MELD scores who receive LT. Furthermore, the higher proportion of hepatocellular carcinoma observed in the ABOi-LDLT group, compared with the DDLT and ABOc-LDLT groups, suggested that they had a relatively overall better health condition regardless of MELD score. This result was consistent with the fact that patients who undergo LT as treatment for hepatocellular carcinoma generally tend to have a more favorable health status than those with end-stage liver disease (43). We mitigated this concern by conducting multivariate logistic regression analysis and using propensity score matching to account for confounding factors.
Except for biliary stricture, the complication rates among LT recipients were similar. Biliary stricture occurred in 25% of the ABOi-LDLT group recipients and 10% of the DDLT group recipients. Although use of ABOi-LDLT is associated with a high prevalence of diffuse intrahepatic biliary stricture, compared with ABOc-LDLT, our results did not demonstrate significant biliary stricture rates (15). When we investigated a larger dataset from our institution, irrespective of MELD scores, we found no difference between recipients of ABOi-LDLT vs. ABOc-LDLT (Appendix 13).
Among patients who received ABOi-LDLT, we found an elevated rate of in-hospital mortality and graft loss within the first year in those with higher initial isoagglutinin titers, compared with recipients with lower titers. Infection was the leading factor associated with mortality within this period; it accounted for the majority of the deaths (62.5%), which was consistent with previous research involving all LDLT recipients (44). Recipients in the high initial isoagglutinin titer group tended to receive a comparatively higher dosage of rituximab and underwent more rounds of TPE. The high mortality rates observed in recipients with high initial isoagglutinin titer may lead to a debate over whether to opt for ABOi-LDLT as opposed to DDLT. However, in regions facing organ shortage, the waiting time for DDLT is often too long. In South Korea, the national average waiting time for all patients, irrespective of their MELD score, was reported to be approximately 280 days, from 2011 to 2020 (23). This prolonged waiting time lead to increased waiting mortality, which providing a rationale to consider ABOi-LDLT for patients even with high initial isoagglutinin titers.
We found a lack of correlation between initial isoagglutinin titer and rejection rate. Unlike previous studies, we also found no correlation between initial isoagglutinin titer and isoagglutinin titer rebound (45,46). There was no biopsy-proven antibody-mediated rejection in recipients of ABOi-LDLT. Despite our effort to find other factors associated with the rebound of isoagglutinin titer, there were no differences in preoperative characteristics, graft loss, or rejection rates between the two groups (i.e., those who had a rebound of isoagglutinin titer vs. those who did not) (Appendix 11). Therefore, our results indicated that initial isoagglutinin titer and isoagglutinin titer rebound had no association with post-transplant outcomes in ABOi-LDLT recipients with a MELD score ≥30.
The retrospective nature of this study could have led to the difference between the ITT-ABOi-LDLT and the other two groups; it is one limitation of this study. However, we maximized the similarity between groups by adjusting clinical variables at the time of waitlisting with MELD scores ≥30 and including the types and numbers of organ failure on an ITT basis. The single-center data was another limitation because indication and desensitization protocols for ABOi-LDLT can vary among centers. Lastly, the survival benefit of ABOi-LDLT in patients with high MELD scores could be applied differently, according to region-associated differences in organ shortage severity. Further studies that include multicenter data from various regions are required.
Conclusions
The ITT analyses used in this study found that compared with waiting for DDLT, ABOi-LDLT had a considerable survival benefit for patients with MELD scores ≥30. Recipients of ABOi-LDLT had post-transplant outcomes comparable to those who underwent DDLT or even ABOc-LDLT. ABOi-LDLT is a feasible treatment option for patients with a high MELD score in areas experiencing critical organ shortages, particularly when the only available living donor is ABOi.
Acknowledgments
Medical Illustration & Design (MID), as a member of the Medical Research Support Services of Yonsei University College of Medicine, providing excellent support with medical illustration.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-58/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-25-58/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Severance Hospital (No. 4-2022-0913), and individual consent for this retrospective analysis was waived because of its retrospective design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015;15:17-38. [Crossref] [PubMed]
- Goldaracena N, Barbas AS. Living donor liver transplantation. Curr Opin Organ Transplant 2019;24:131-7. [Crossref] [PubMed]
- Yoshizumi T, Taketomi A, Soejima Y, et al. Impact of donor age and recipient status on left-lobe graft for living donor adult liver transplantation. Transpl Int 2008;21:81-8. [PubMed]
- Yi NJ, Suh KS, Lee HW, et al. Improved outcome of adult recipients with a high model for end-stage liver disease score and a small-for-size graft. Liver Transpl 2009;15:496-503. [Crossref] [PubMed]
- Liu CL, Lam B, Lo CM, et al. Impact of right-lobe live donor liver transplantation on patients waiting for liver transplantation. Liver Transpl 2003;9:863-9. [Crossref] [PubMed]
- Miller CM, Quintini C, Dhawan A, et al. The International Liver Transplantation Society Living Donor Liver Transplant Recipient Guideline. Transplantation 2017;101:938-44. [Crossref] [PubMed]
- Gugenheim J, Samuel D, Reynes M, et al. Liver transplantation across ABO blood group barriers. Lancet 1990;336:519-23. [Crossref] [PubMed]
- Gordon RD, Iwatsuki S, Esquivel CO, et al. Liver transplantation across ABO blood groups. Surgery 1986;100:342-8. [PubMed]
- Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation 2002;73:1959-61. [Crossref] [PubMed]
- Yadav DK, Hua YF, Bai X, et al. ABO-Incompatible Adult Living Donor Liver Transplantation in the Era of Rituximab: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2019;2019:8589402. [Crossref] [PubMed]
- Egawa H, Ohdan H, Saito K. Current Status of ABO-incompatible Liver Transplantation. Transplantation 2023;107:313-25. [Crossref] [PubMed]
- Hsu SC, Thorat A, Jeng LB, et al. ABO-Incompatible Living Donor Liver Transplantation with Reduced Rituximab Dose: A Retrospective Analysis of 65 Patients - Can We Fast-Track Liver Transplant Surgery and Improve Long-Term Survival? Ann Transplant 2020;25:e923502. [Crossref] [PubMed]
- Demetris AJ, Jaffe R, Tzakis A, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol 1988;132:489-502. [PubMed]
- Tanaka A, Tanaka K, Kitai T, et al. Living related liver transplantation across ABO blood groups. Transplantation 1994;58:548-53. [Crossref] [PubMed]
- Song GW, Lee SG, Hwang S, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol 2014;61:575-82. [Crossref] [PubMed]
- Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant 2014;14:102-14. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, et al. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol 2013;59:1215-22. [Crossref] [PubMed]
- Lee CF, Cheng CH, Wang YC, et al. Adult Living Donor Liver Transplantation Across ABO-Incompatibility. Medicine (Baltimore) 2015;94:e1796. [Crossref] [PubMed]
- Györi GP, Silberhumer GR, Zehetmayer S, et al. Dynamic changes in MELD score not only predict survival on the waiting list but also overall survival after liver transplantation. Transpl Int 2012;25:935-40. [Crossref] [PubMed]
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91-6. [Crossref] [PubMed]
- Ravaioli M, Lai Q, Sessa M, et al. Impact of MELD 30-allocation policy on liver transplant outcomes in Italy. J Hepatol 2022;76:619-27. [Crossref] [PubMed]
- Andraus W. Liver transplantation in Brazil: the urgent need of a new allocation system for the exceptions in meld score. Arq Gastroenterol 2020;57:1-2. [Crossref] [PubMed]
- Yim SH, Kim DG, Kang M, et al. Survival benefit of living-donor liver transplantation in patients with a model for end-stage liver disease over 30 in a region with severe organ shortage: a retrospective cohort study. Int J Surg 2023;109:3459-66. [Crossref] [PubMed]
- Lee WC, Cheng CH, Lee CF, et al. Quick preparation of ABO-incompatible living donor liver transplantation for acute liver failure. Clin Transplant 2022;36:e14555. [Crossref] [PubMed]
- Yim SH, Min EK, Choi MC, et al. Unusual grafts for living-donor liver transplantation. Eur J Med Res 2023;28:454. [Crossref] [PubMed]
- Lee J, Lee JG, Lee JJ, et al. Results of ABO-incompatible liver transplantation using a simplified protocol at a single institution. Transplant Proc 2015;47:723-6. [Crossref] [PubMed]
- Kim WR, Mannalithara A, Kwo PY, et al. Mortality in patients with end-stage liver disease above model for end-stage liver disease 3.0 of 40. Hepatology 2023;77:851-61. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047-81. [Crossref] [PubMed]
- Perez Ruiz de Garibay A, Kortgen A, Leonhardt J, et al. Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit Care 2022;26:289. [Crossref] [PubMed]
- Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet 2015;386:1576-87. [Crossref] [PubMed]
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361:1279-90. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Wong TC, Fung JY, Pang HH, et al. Analysis of Survival Benefits of Living Versus Deceased Donor Liver Transplant in High Model for End-Stage Liver Disease and Hepatorenal Syndrome. Hepatology 2021;73:2441-54. [Crossref] [PubMed]
- Lee WC, Lee CS, Wang YC, et al. Validation of the Model for End-Stage Liver Disease Score Criteria in Urgent Liver Transplantation for Acute Flare Up of Hepatitis B. Medicine (Baltimore) 2016;95:e3609. [Crossref] [PubMed]
- Kim JY, Lee HS, Chung MJ, et al. Bleeding Complications and Clinical Safety of Endoscopic Retrograde Cholangiopancreatography in Patients with Liver Cirrhosis. Yonsei Med J 2019;60:440-5. [Crossref] [PubMed]
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397:2212-24. [Crossref] [PubMed]
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11-20. [Crossref] [PubMed]
- Battistella S, D'Arcangelo F, Grasso M, et al. Liver transplantation for non-alcoholic fatty liver disease: indications and post-transplant management. Clin Mol Hepatol 2023;29:S286-301. [Crossref] [PubMed]
- Bodzin AS, Baker TB. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transpl 2018;24:1470-5. [Crossref] [PubMed]
- Larsen FS, Schmidt LE, Bernsmeier C, et al. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol 2016;64:69-78. [Crossref] [PubMed]
- Tan EX, Wang MX, Pang J, et al. Plasma exchange in patients with acute and acute-on-chronic liver failure: A systematic review. World J Gastroenterol 2020;26:219-45. [Crossref] [PubMed]
- David S, Bode C, Putensen C, et al. Adjuvant therapeutic plasma exchange in septic shock. Intensive Care Med 2021;47:352-4. [Crossref] [PubMed]
- Hung HC, Lee JC, Wang YC, et al. Living-Donor Liver Transplantation for Hepatocellular Carcinoma: Impact of the MELD Score and Predictive Value of NLR on Survival. Curr Oncol 2022;29:3881-93. [Crossref] [PubMed]
- Kim SI, Kim YJ, Jun YH, et al. Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med J 2009;50:112-21. [Crossref] [PubMed]
- Lee WC, Lee CF, Wu TH, et al. Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation. J Pers Med 2021;11:1300. [Crossref] [PubMed]
- Lee EC, Kim SH, Shim JR, et al. A comparison of desensitization methods: Rituximab with/without plasmapheresis in ABO-incompatible living donor liver transplantation. Hepatobiliary Pancreat Dis Int 2018;17:119-25. [Crossref] [PubMed]


