
Prognostic factors in gallbladder cancer: a comprehensive systematic review and meta-analysis
Highlight box
Key findings
• Key factors impacting gallbladder cancer (GBC) outcomes included lymphocyte-monocyte ratio (LMR), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA199) levels, lymph node status, T stage, invasion presence, tumor location, and surgical margin status. Notably, chemotherapy and radiotherapy significantly improved overall survival. However, jaundice and gallstones did not independently predict outcomes, suggestive of advanced-stage symptoms.
What is known and what is new?
• GBC, known for its aggressiveness and increasing prevalence due to improved diagnostics, poses challenges in prognosis and treatment, primarily managed with surgery despite high recurrence and low survival rates.
• Our study revealed that factors such as LMR, CEA, CA199 levels, lymph node status, T stage, invasion, tumor location, and surgical margin influenced GBC outcomes, with chemotherapy and radiotherapy showing survival benefits.
What is the implication, and what should change now?
• This study consolidates risk factors impacting prognosis, offering valuable insights for patients with poor prognoses. Future research should delve deeper into understanding the underlying mechanisms of these prognostic factors.
Introduction
Gallbladder cancer (GBC), as the most prevalent malignancy among biliary tract cancers, contributes to an escalating global health burden in Asia and South America (1). Historically, GBC has been recognized as a highly aggressive and malignant disease. With the advancement of diagnostic tools, there has been a noticeable increase in the detection of incident GBC cases over the past few decades, necessitating a more serious consideration of this disease. Recent reports have shown a significant rise in the number of incidental GBC cases, exceeding 5,000 incident cases annually, accompanied by more than 4,000 deaths (2). The phenomenon has elevated GBC to become the third most common cancer within the gastrointestinal tract, particularly in Asia (3).
However, the notoriety of GBC is not solely attributed to its prevalence; rather, its insidious nature and the absence of early symptoms and signs often result in delayed diagnoses, leading to the disease being diagnosed at an advanced stage. Additionally, the anatomical location of the gallbladder, lacking a serosal covering on its hepatic side, predisposes it to lymph node metastasis and liver involvement, further complicating the prognosis (4). Unfortunately, at the time of presentation, fewer than 35% of these cancers are amenable to surgical resection.
Currently, the primary therapeutic approach for GBC remains surgical resection. However, even in cases where patients undergo radical resection, they still confront the harsh reality of a high recurrence rate, ranging from 46% to 61%, and a dismal overall survival (OS) rate, often falling below 15% (5,6). Consequently, beyond surgical resection, several alternative treatment methods, including chemotherapy, radiotherapy, and immune therapy, have emerged as potential strategies. The challenge lies in comprehensively identifying all factors that may influence the prognosis of GBC, facilitating the development of more efficient treatment protocols while avoiding the risk of over-treatment. Prognosis in this context is primarily defined by the 5-year OS rate following curative-intent surgery for GBC.
Between 2015 and 2021, many meta-analyses on GBC prognosis were published; however, none comprehensively analyzed the post-curative-intent surgery survival of GBC patients. One of these studies, due to its earlier publication date, did not include or analyze the latest available data (7). Some meta-analyses have highlighted adjunctive treatment methods for GBC patients (8,9), while others have predominantly examined surgical treatment modalities (10,11). Additionally, certain studies have specifically analyzed preoperative indicators (12). Moreover, some studies have narrowed their focus to patients presenting with jaundice (13) or T2 stage disease (14).
Due to the lack of a consensus regarding prognostic factors influencing GBC outcomes, this study aims to elucidate and identify individual factors associated with GBC prognosis and their relationships to outcomes through a systematic review. We present this article in accordance with the PRISMA reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-502/rc) (15).
Methods
Study design and registration
The systematic review protocol was registered on PROSPERO with the corresponding ID: CRD42022352675.
Search strategy
The literature search and data extraction processes were independently carried out by two authors, X.H. and N.W. Multiple databases, including PubMed, Cochrane Library, and Embase, were explored using the following keywords and terms: “gallbladder neoplasm” OR “gallbladder carcinoma” OR “gall bladder cancer” AND “prognostic factors”. The inclusion criteria encompassed studies focusing on patients who had undergone curative-intent surgery. The search was restricted to articles published between 2000 and July 31, 2023, and only English-language articles were included.
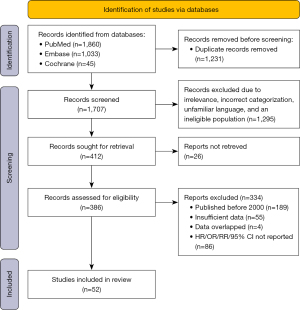
After eliminating invalid article types and duplicates, the title and abstract of each remaining article were reviewed to identify potentially relevant studies. Subsequently, full-text versions of these articles were retrieved, and data were meticulously extracted. In cases where discrepancies arose regarding the inclusion of specific texts, we engaged D.Z. to aid in resolving the matter. The whole process is displayed in Figure 1.
Selection criteria
Studies meeting the following criteria will be included in our research. The study centered on patients who underwent curative-intent surgery for gallbladder carcinoma or neoplasm and provided a report on the prognosis of included patients. Articles were published after the year 2000. Data on hazard ratios (HRs), relative ratios (RRs), or odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were available, or there was sufficient data to calculate the standard error (SE).
Studies with the following conditions will be excluded from our research. Studies were excluded if they involved patients with unresectable tumors. Studies lacking sufficient data, including those without reported postoperative survival times or essential statistics such as HR, OR, or RR, were excluded from the analysis. Previous reviews, case reports, editorials, letters, correspondence, conference abstracts, and animal studies were also excluded.
Data extraction
Data were extracted in the following order: first author, publication year, country of study, study design, OS rate, surgical characteristics, TNM stage, tumor differentiation, lymph node metastasis, surgical margin status, invasion, adjuvant treatment, complications, HR, OR, or RR with 95% CI. An HR greater than 1 indicated poorer survival in comparison to the experimental group.
Quality assessment
The quality of the included studies was assessed by two authors, X.H. and N.W., using the Newcastle-Ottawa Scale (NOS) tool displayed in Table 1. This tool comprises eight domains that assess the validity of articles and the risk of bias: representativeness of the exposed cohort, Selection of the non-exposed cohort, Ascertainment of exposure, demonstration that outcome of interest was not present at start of study, comparability of cohorts on the basis of the design or analysis, assessment of outcome, was follow-up long enough for outcomes to occur, adequacy of follow up of cohorts.
Table 1
| Author | Year | Number of cases | Journal |
|---|---|---|---|
| Tian-Run Lv (16) | 2023 | 255 | J Gastrointest Surg |
| Kizuki Yuza (17) | 2021 | 200 | Langenbecks Arch Surg |
| Yuri Jeong (18) | 2014 | 86 | Anticancer Res |
| Chuan You (19) | 2022 | 102 | BMC Surg |
| Jonathan Navarro (20) | 2019 | 100 | J Surg Res |
| Sae Byeol Choi (21) | 2010 | 83 | J Gastrointest Surg |
| Hyun Lim (22) | 2013 | 279 | J Clin Gastroenterol |
| John R Bergquist (23) | 2018 | 4,373 | Int J Surg |
| Yuhree Kim (24) | 2016 | 291 | Ann Surg Oncol |
| W Kwon (25) | 2020 | 937 | Br J Surg |
| Kelly Lafaro (26) | 2020 | 1,251 | J Surg Oncol |
| Junichi Shindoh (27) | 2015 | 438 | Ann Surg |
| Fei Liu (28) | 2019 | 90 | Medicine (Baltimore) |
| Weiyu Xu (29) | 2020 | 154 | Cancer Manag Res |
| Yoshio Shirai (30) | 2012 | 135 | World J Surg Oncol |
| Masashi Utsumi (31) | 2021 | 116 | BMC Gastroenterol |
| Lejia Sun (32) | 2021 | 140 | J Cancer |
| Wei-Yu Xu (33) | 2018 | 154 | World J Gastroenterol |
| Andrew M Blakely (34) | 2019 | 66 | J Surg Oncol |
| Wen-Yan Yao (35) | 2022 | 104 | Hepatobiliary Pancreat Dis Int |
| Cecilia G Ethun (36) | 2018 | 445 | Am Surg |
| Wan-Joon Kim (37) | 2020 | 132 | Cancer Control |
| Woohyung Lee (38) | 2017 | 192 | Surgery |
| Huisong Lee (39) | 2015 | 157 | Ann Surg Oncol |
| Hiroshi Yagi (40) | 2006 | 63 | J Hepatobiliary Pancreat Surg |
| Rui-Tao Wang (41) | 2015 | 223 | World J Gastroenterol |
| Mehmet Ali Uzun (42) | 2022 | 64 | Turk J Surg |
| Myongjin Kim (43) | 2021 | 539 | Cancers (Basel) |
| Sundeep Singh Saluja (44) | 2022 | 100 | J Gastrointest Surg |
| Zainab Feroz (45) | 2022 | 176 | World J Surg Oncol |
| Sameer Gupta (46) | 2022 | 115 | J Surg Oncol |
| Xiwei Cui (47) | 2018 | 159 | Medicine (Baltimore) |
| Yan Deng (48) | 2019 | 169 | Cancer Manag Res |
| Sha Zhu (49) | 2019 | 255 | Sci Rep |
| Xabier de Aretxabala (50) | 2006 | 139 | J Gastrointest Surg |
| Jin-Kyu Cho (51) | 2019 | 81 | World J Surg Oncol |
| Huifang Dai (52) | 2022 | 202 | BMC Surg |
| Li Wang (53) | 2018 | 307 | Oncol Lett |
| Christina Y Koh (54) | 2013 | 42 | Am Surg |
| Hao Chen (55) | 2021 | 93 | J Surg Oncol |
| Wei Zhang (56) | 2021 | 1,009 | Front Oncol |
| Dong-Xu Fan (57) | 2018 | 893 | World J Gastroenterol |
| SH Kim (58) | 2016 | 128 | Eur J Surg Oncol |
| Yan Deng (59) | 2016 | 315 | Tumour Biol |
| Yongjin Bao (60) | 2021 | 144 | Biosci Trends |
| Sunil Choudhary (61) | 2015 | 33 | J Clin Diagn Res |
| Rui-Qi Zou (62) | 2022 | 50 | Front Oncol |
| Mee Joo Kang (63) | 2012 | 421 | HPB (Oxford) |
| Se-Il Go (64) | 2016 | 84 | Cancer Res Treat |
| Zhihang Tao (65) | 2018 | 84 | Cancer Biomark |
| Qing Pang (66) | 2015 | 316 | World J Gastroenterol |
| Y Nakakubo (67) | 2003 | 45 | Br J Cancer |
Statistical analysis
To explore factors affecting patient prognosis, we will aggregate and analyze variables reported in five or more studies to assess their prognostic significance. These factors were classified into three main categories: biomarkers and clinical indices, clinical-pathological conditions, and treatment-related factors. Data were extracted and recorded in an Excel spreadsheet. Subsequently, STATA (version 17.0) was employed to calculate and analyze the relevant data, which had been transformed logarithmically from HR values. During this process, the 95% CI corresponding to each HR value will also be calculated. In cases where HR values were unavailable and other necessary data were lacking for SE calculation, we utilized the method proposed by Tierney, which involves digitizing data from Kaplan-Meier curves (68).
Data heterogeneity was assessed using the Cochran Q statistic and the I2 statistic, representing the proportion of total variation attributed to heterogeneity (69). In cases where substantial heterogeneity was detected (I2>50%), a random-effects model was applied (70). Otherwise, a fixed-effects model was employed. Forest plots were generated to visualize the data’s effects, while funnel plots were used to evaluate the potential influence of publication bias. Sensitivity analysis was conducted by sequentially excluding each individual study to assess whether any particular study had a significant impact on the pooled point estimate and CI (71).
Through this systematic approach, we aim to conduct a thorough and accurate analysis of all included prognostic factors, identifying those associated with patient prognosis. Our goal is to uncover prognostic factors that benefit patient outcomes, ultimately deriving meaningful conclusions and findings.
Results
The initial search yielded 2,938 potential studies. After removing 1,231 duplicates, the remaining studies underwent a rigorous selection process. Among them, 162 were case studies, 11 were systematic reviews, 836 had irrelevant content, 301 involved ineligible populations, 11 were written in unfamiliar languages, 189 were published before 2000, 15 were unreadable, and 127 full-text articles were excluded for various reasons. Eventually, 57 studies met the inclusion criteria, and after further evaluation, 52 studies were deemed suitable for analysis. The basic characteristics of the included studies are presented in Table 2. The reference list of studies included in the meta-analysis is available in Table 1.
Table 2
| Study | Selection | Comparability | Outcome | NOS score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts based on the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||||
| Tian-Run Lv (16), 2023 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Myongjin Kim (43), 2021 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Kizuki Yuza (17), 2021 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | – | 8 | ||
| Yuri Jeong (18), 2014 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Sundeep Singh Saluja (44), 2022 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Chuan You (19), 2022 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Sunil Choudhary (61), 2015 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Zainab Feroz (45), 2022 | ★ | ★ | ★ | ★ | ★★ | – | ★ | ★ | 8 | ||
| Rui-Qi Zou (62), 2022 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Sameer Gupta (46), 2022 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Jonathan Navarro (20), 2019 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Xiwei Cui (47), 2018 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Mee Joo Kang (63), 2012 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Sae Byeol Choi (21), 2010 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | 7 | ||
| Wan-Joon Kim (37), 2020 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | 7 | ||
| John R Bergquist (23), 2018 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Se-Il Go (64), 2016 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Yuhree Kim (24), 2016 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| W Kwon (25), 2020 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Kelly Lafaro (26), 2020 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Woohyung Lee (38), 2017 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Junichi Shindoh (27), 2015 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Huisong Lee (39), 2015 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Yan Deng (48), 2019 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Fei Liu (28), 2019 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Sha Zhu (49), 2019 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Zhihang Tao (65), 2018 | ★ | ★ | ★ | ★ | ★★ | – | ★ | ★ | 8 | ||
| Hiroshi Yagi (40), 2006 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Weiyu Xu (29), 2020 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Qing Pang (66), 2015 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Rui-Tao Wang (41), 2015 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Hyun Lim (22), 2013 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Mehmet Ali Uzun (42), 2022 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | 7 | ||
| Wei Zhang (56), 2021 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | 7 | ||
| Dong-Xu Fan (57), 2018 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| SH Kim (58), 2016 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Yoshio Shirai (30), 2012 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Xabier de Aretxabala (50), 2006 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | 7 | ||
| Jin-Kyu Cho (51), 2019 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Huifang Dai (52), 2022 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Masashi Utsumi (31), 2021 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Yan Deng (59), 2016 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Li Wang (53), 2018 | ★ | ★ | ★ | ★ | ★★ | – | ★ | ★ | 8 | ||
| Christina Y Koh (54), 2013 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Yongjin Bao (60), 2021 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Lejia Sun (32), 2021 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Wei-Yu Xu (33), 2018 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | 7 | ||
| Andrew M Blakely (34), 2019 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||
| Wen-Yan Yao (35), 2022 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Cecilia G Ethun (36), 2018 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Hao Chen (55), 2021 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
| Y Nakakubo (67), 2003 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | ||
NOS, Newcastle-Ottawa Scale.
These 52 studies encompassed a collective cohort of 23,174 patients, with a gender distribution ratio of 38:62 (male to female). The reported follow-up periods ranged from 20 months to 60 months. All the included studies exhibited high methodological quality, and the use of the Quality in Prognostic Factor Studies (QUIPS) tool indicated that these studies had a low risk of bias.
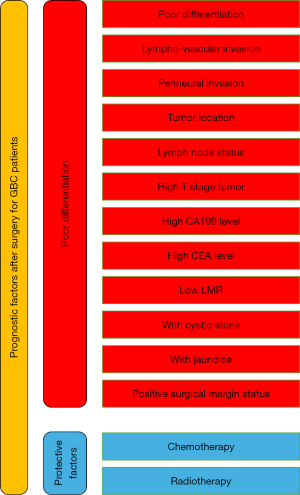
The prognostic factors under investigation were assessed in terms of their impact on mortality after resection, with all eligible risk or protective factors analyzed. These factors were categorized into three main groups: biomarkers and clinical indices, clinical-pathological conditions, and treatment-related factors. The key findings of our study are presented in Figure 2.
Biomarkers/clinical indices
In the category of biomarkers and clinical indices, three factors were considered: lymphocyte-monocyte ratio (LMR), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA199).
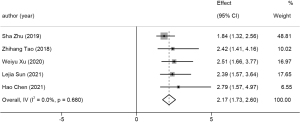
LMR <0.3
Five studies reported a reduced 5-year OS rate in patients with an LMR less than 0.3. This group comprised 726 cases, and the pooled HR was 2.17 (95% CI: 1.73 to 2.60, P<0.001, I2=0.0%). A forest plot is available in Figure 3.
CEA >5 ng/mL
Several studies investigated the association between OS rate and patients with CEA levels exceeding 5 ng/mL. A total of 1,915 patients were included in this category, with an HR of 1.81 (95% CI: 1.44 to 2.18, P<0.001, I2=0.0%). A forest plot is available in Figure 4.
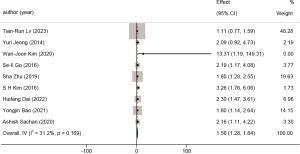
CA199 >37 U/mL
Using the common CA199 threshold of 37 U/mL, nine studies enrolled a total of 1,154 patients in this category. The HR was 1.56 (95% CI: 1.28 to 1.84, P<0.001, I2=31.2%). A forest plot is available in Figure 5.
Clinical-pathological conditions
In this category, various clinical-pathological conditions that serve as universal prognostic factors in cancers were analyzed. The factors considered included differentiation, the presence of invasion, tumor location, node status, and T-stage.
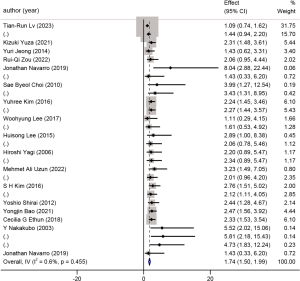
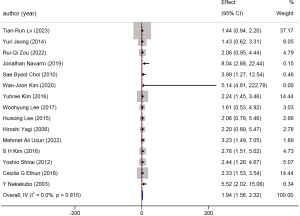
Presence of invasion
A total of 18 studies with 2,670 patients were included in the analysis of the presence of invasion. A forest plot is shown in Figure 6. This factor was further subdivided into lymph-vascular invasion and perineural invasion. The HR for lymph-vascular invasion was 1.94 (95% CI: 1.56 to 2.32, P<0.001, I2=0.0%) (Figure 7), while the HR for perineural invasion was 1.58 (95% CI: 1.25 to 1.91, P<0.001, I2=16.2%) (Figure 8).
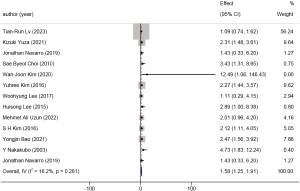
Tumor location
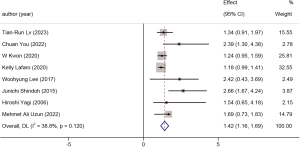
Eleven studies explored the relationship between different tumor locations and the prognosis of GBC, involving 3,814 patients. Patients with tumors located closer to the hepatic side had an HR of 1.42 (95% CI: 1.16 to 1.69, P=0.001, I2=38.8%) compared to those with peritoneal-side tumors. A forest plot is available in Figure 9.
Node status
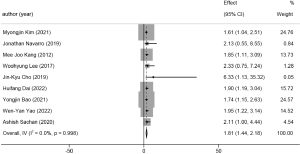
Thirty-two studies related to lymph node status were combined, including a total of 12,194 cases. The pooled HR for the presence of lymph node metastasis was 2.03 (95% CI: 1.75 to 2.31, P<0.001, I2=67.3%). A forest plot is available in Figure 10.
T-stage
Fourteen studies involving 7,955 patients were included to assess the impact of T-stage on prognosis. The population was divided into two groups: early stage (T1/T2) and advanced stage (T3/T4). The pooled HR for advanced-stage tumors was 2.37 (95% CI: 1.98 to 2.75, P=0.001, I2=63.0%). A forest plot is available in Figure 11.
Differentiation
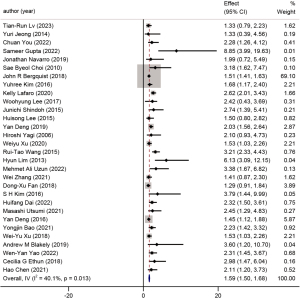
Thirty studies reported on differentiation as a prognostic factor in GBC, with a cumulative patient population of 12,761. Patients with well and moderately well-differentiated tumors were compared to those with poorly differentiated tumors. The HR for poorly differentiated tumors was 1.59 (95% CI: 1.50 to 1.68, P=0.01, I2=40.1%). A forest plot is available in Figure 12.
Presence of preoperational jaundice
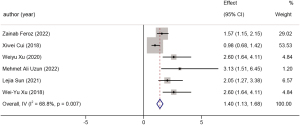
Six studies examined the relationship between the presence of preoperational jaundice and the 5-year OS rate of GBC patients. A total of 847 patients were included in this analysis. The HR was 1.40 (95% CI: 1.13 to 1.68, P=0.007, I2=68.8%). A forest plot is available in Figure 13.
Presence preoperational of gallstone
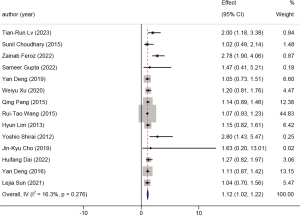
Fourteen studies were included in the analysis of the presence of gallstones. The total population in this analysis was 2,593. The HR was 1.12 (95% CI: 1.02 to 1.22, P<0.001, I2=16.3%). A forest plot is available in Figure 14. The presence of gallstones may affect GBC prognosis through two pathways: mechanical stimulation and inflammation induced by gallstones, and bacterial colonization leading to bile acid degradation and chronic mucosal damage, both contributing to carcinogenesis (72).
Treatment-related factors
While surgical resection remains the primary treatment for GBC, the roles of chemotherapy and radiotherapy in improving patient outcomes have been subjects of debate. Therefore, three treatment-related factors were analyzed: surgical margins, chemotherapy, and radiotherapy.
Surgical margins
Twenty-one studies reported on surgical margins as a potential risk factor for the prognosis of GBC. A total of 9,799 GBC patients who underwent curative-intent resection were included. Due to anatomical reasons, R0 resection was not possible for some patients. A comparison between patients who received R0 resection and those who received R1 or R2 resection revealed an HR of 2.66 (95% CI: 2.19 to 3.12, P<0.001, I2=70.2%). A forest plot is available in Figure 15.

Chemotherapy
Eighteen studies were identified that involved GBC patients who underwent curative-intent resection and received chemotherapy. The cumulative patient population in this dataset was 8,478. The pooled HR was 0.75 (95% CI: 0.70 to 0.80, P<0.001, I2=61.4%). A forest plot is available in Figure 16.
Radiotherapy
Seven studies reported on the prognosis of GBC patients who received radiotherapy after surgery. A total of 2,923 patients were involved in this analysis. The pooled HR was 0.56 (95% CI: 0.47 to 0.64, P<0.001, I2=61.8%). A forest plot is available in Figure 17.
Evaluation of publication bias
Egger tests were conducted to assess the risk of publication bias for each analysis of prognostic factors. The outcomes of the Egger tests indicated a low risk of publication bias, as shown by the Egger graph in Figure S1.
Sensitivity analysis
We conducted sensitivity analysis using StataMP 17 software (StataCorp. 2022. Stata Statistical Software: Release 17), and the results affirmed the reliability of our conclusions. Additionally, the sensitivity analysis for each prognostic factor is detailed in the Figure S2.
Subgroup analysis
To assess potential influences of study duration and racial variations on the data, subgroup analyses were performed based on years and countries. The relevant results can be found in Figure S3. Apart from surgical margins, chemotherapy, and gallstones, the majority of factors showed no significant association with country variations. Furthermore, none of the prognostic factors exhibited statistically significant differences between time groups. However, due to data constraints, subgroup analysis was not feasible for radiotherapy and LMR. The results of subgroup analysis indicate that the geographical location of the studies may contribute to the heterogeneity of the analysis results, but overall, the conclusions remain reliable.
Discussion
GBC is a rare and aggressive malignancy, primarily affecting elderly individuals. It often remains undiagnosed until it reaches an advanced stage, leading to poor prognosis. The median survival for GBC is only about 6 months, and the reported 5-year survival rate is as low as 5% (73). However, when early-stage cancers are incidentally discovered during cholecystectomy for gallstones, the 5-year survival rate exceeds 80% (74). This fact highlights the importance of early diagnosis, as GBC, although uncommon, has been on the rise in recent decades and poses significant challenges in terms of diagnosis and management (14).
In this systematic review, the primary studies were predominantly from Asian countries, underscoring the wide prevalence of GBC in Asia. Our goal was to comprehensively investigate the numerous factors influencing the overall survival (OS) of GBC patients.
This study represents the most comprehensive analysis of complex prognostic factors for gallbladder carcinoma, encompassing data from 57 studies and 23,174 cases. Ultimately, we identified 11 risk or protective factors for the prognosis of GBC.
Biomarkers/clinical indices
As our understanding of immune indicators deepens, there has been a growing focus on this subject in research. Several previous studies have reported various indices such as the neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR). However, due to the absence of consensus on standard criteria for immune indicators, we were unable to pool NLR and PLR data in our analysis.
In this study, we successfully extracted data on the LMR, the only immune indicator we could include from earlier studies. In the studies included in our analysis, blood specimens were collected preoperatively after diagnosis but before surgery, from which the LMR was calculated by dividing the total lymphocyte count by the total monocyte count. LMR has been reported to play a significant role in various types of cancers and represents a potential and promising direction for research. A decreased LMR indicates lymphopenia resulting from an insufficient immunologic response to tumors and heightened levels of monocytes and macrophages in the tumor microenvironment, fostering cancer advancement and hampering antitumor immune response. Our findings align with previous research (75), which has consistently indicated that lower lymphocyte counts are associated with poorer outcomes (76), likely due to the crucial role of lymphocytes in the body’s anti-tumor defense mechanisms. Further research is needed in the future to elucidate the specific functioning of anti-tumor defense in individual cancers.
Furthermore, we analyzed CA199 and CEA, which are widely used indicators. We divided the population into high and low-risk groups based on these markers. Our findings indicate that a CA199 level greater than 37 U/mL was associated with an HR of 1.56 (95% CI: 1.28 to 1.84, P<0.001, I2=31.2%), confirming that elevated CA199 predicts lower survival (77). Similarly, a CEA level greater than 5 ng/mL had a higher HR of 1.81 (95% CI: 1.44 to 2.18, P<0.001, I2=0.00%), highlighting that CEA reflects survival more definitively. Traditionally, elevated levels of CA199 and CEA are not only associated with the presence of metastatic disease in GBC patients (78), but are also considered potential predictors of recurrence (79). The higher odds of recurrence and metastatic disease explain the lower survival rates among GBC patients with high CA199 or CEA levels (80).
Clinical-pathological conditions
Among the identified risk factors, the majority were associated with clinical-pathological conditions. Here, we summarize the findings for five key individual factors, including T-stage, lymph node status, invasion, location, and other factors that interfere with prognosis.
T-stage
T-stage emerged as the most critical factor in prognosis, with an HR of 2.37. This indicates that patients with T3 or T4 stage tumors have a mortality rate exceed to twice that of patients with T1 or T2 stage tumors. T-stage is a pivotal determinant of clinical stage, and the surgical approach and resection width often depend on the T-stage.
Lymph node status
Several studies have suggested that disease-specific survival and OS of node-negative patients are significantly better than those of node-positive patients (81). To provide evidence for this statement, we conducted a comprehensive analysis of numerous studies and found that node-positive patients had an HR of 2.03, confirming that lymph node involvement is a significant predictor of worse prognosis.
Invasion
We investigated the impact of gallbladder tumor invasion, including lymph-vascular invasion and perineural invasion. The combined HR for all invasion events was 1.74. Subgroup analysis revealed that lymph-vascular invasion had a higher HR of 1.94 compared to perineural invasion, which had HR of 1.58. The anatomical features of lymph-vascular invasion make it particularly fatal, as it is challenging to resect and more likely to result in sinus metastasis.
Location
Tumor location within the gallbladder was identified as an individual prognostic element for GBC. Patients with tumors located on the side of the liver had a higher HR for 5-year OS. This could be attributed to the fact that tumors on the side of the liver were strongly correlated with a higher incidence of lymph node metastasis and perineural invasion and were marginally correlated with a higher rate of recurrence, resulting in worse survival compared with the survival of the patients with peritoneal side tumor (82).
Other factors
In addition to the factors mentioned above, other elements interfering with prognosis included the presence of jaundice (HR =1.40), the presence of gallstones (HR =1.12), and tumor differentiation (HR =1.59). Empirically, preoperative jaundice presence was highly correlated with advanced stage (III and IV) in our patient subset, making it inappropriate to consider jaundice as an independent predictor of 5-year OS rate (83). The presence of gallstones was associated with mortality, but this result might be influenced by silent gallstones, which patients often neglect for an extended period (72). Differentiation was categorized as well/medium differentiation versus poor differentiation, with the latter having a higher HR in 5-year OS. However, the lack of a unified definition for specific criteria prevented further detailed analysis.
These clinical-pathological factors play crucial roles in predicting the prognosis of GBC, and understanding their impact is vital for patient management and treatment decisions.
Treatment-related factors
In the final analysis of treatment-related factors, three significant factors were identified: surgical margins, chemotherapy, and radiotherapy.
Surgical margins
Surgical margins were found to be a crucial factor in the prognosis of GBC. Patients who received R1/R2 resection, indicating that the tumor was not completely removed during surgery, showed an inferior OS rate, with an HR of 2.66. Empirically, invasion events usually bonded with unresectable tumor together, which meant patients had no choice but received R1 or R2 resection, because disruption of the natural barriers between the tumor and the lymph-vascular network in the liver bed during cholecystectomy may lead to residual cancer cells being trapped in the liver bed (84). Importantly, even when comparing patients with invasion events, those in the surgical margins group had a higher HR of 2.563 compared to the invasion group’s HR of 1.508 (85). This suggests that the extent of surgical resection has a more significant impact on prognosis than the presence of invasion. These findings underscore the importance of extensive resection and aim for R0 resection whenever possible, even in cases where invasion is present.
Chemotherapy and radiotherapy
The effectiveness of adjuvant therapy, including chemotherapy and radiotherapy, has been a subject of debate for GBC. However, this analysis revealed that patients who received chemotherapy or radiotherapy had better prognosis, aligning with previous studies (86). Additionally, the data indicated that radiotherapy had a more critical impact on prognosis than chemotherapy as (87) reported. Adjuvant therapy was shown to improve 5-year OS in GBC patients, especially those with positive resection margins or lymph node involvement (88). Noteworthily, patients who received both chemotherapy and radiotherapy were not removed from the analysis, since subgroup analysis showed no significant difference in this group as displayed in Figure 17.
Clinical applications
Understanding the significance of these factors in clinical settings can be transformative. Firstly, they enable clinicians to stratify patients based on their risk profiles. For instance, patients with advanced TNM stage, lymph node metastasis, or poorly differentiated tumors may benefit from more aggressive treatment strategies and closer postoperative surveillance (89). Conversely, patients with favorable prognostic factors can be spared unnecessary interventions.
Secondly, the incorporation of these factors into clinical decision-making can guide treatment choices. Adjuvant therapy should be tailored to individual risk profiles. Our findings support the use of adjuvant therapy for patients at high risk, as it was associated with significantly improved survival. Conversely, for patients at lower risk, treatment may be more conservatively selected to minimize unnecessary side effects (90).
In summary, this research article comprehensively investigates the various factors influencing the OS of patients with GBC who have undergone curative-intent surgery and offers practical implications for clinical practice. This study’s significance lies in its capacity to consolidate the risk factors affecting prognosis, thereby providing valuable insights for patients with unfavorable prognoses. However, it is essential to remember that individual patient cases may vary, and clinical decisions should always consider the unique characteristics and preferences of each patient.
Limitations
This study has several limitations. Firstly, despite our comprehensive search strategy, there is a possibility of missing relevant studies published in languages other than English. Secondly, although we conducted subgroup analyses by year and country, data heterogeneity may persist due to differences in patient populations and treatment practices across regions and time periods. Thirdly, the included studies may exhibit variations in the definition and assessment of prognostic factors, potentially introducing heterogeneity. Fourthly, our analysis primarily focused on studies conducted in Asian countries. Although the subgroup analysis based on geographic factors revealed no significant heterogeneity in the results, further original research in non-Asian areas is warranted.
Conclusions
GBC remains a challenging malignancy with a generally poor prognosis. This comprehensive systematic review and meta-analysis identified several critical prognostic factors, including clinical-pathological conditions (T stage, lymph-vascular invasion, perineural invasion, tumor location, lymph node status, differentiation, preoperative jaundice, and gallstones), biomarkers/clinical indices (LMR, CEA, and CA199), and treatment-related factors (surgical margins, chemotherapy, and radiotherapy). These factors should be considered when assessing the prognosis of GBC patients and making treatment decisions. However, individual patient characteristics, including age, comorbidities, and overall health, must also be carefully evaluated to provide personalized and tailored treatment plans. Future research should focus on further elucidating the mechanisms underlying these prognostic factors and exploring novel therapeutic strategies to improve outcomes for GBC patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-502/rc
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-502/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-502/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roa JC, García P, Kapoor VK, et al. Gallbladder cancer. Nat Rev Dis Primers 2022;8:69. [Crossref] [PubMed]
- Wu X, Li B, Zheng C, et al. Incidental gallbladder cancer after laparoscopic cholecystectomy: incidence, management, and prognosis. Asia Pac J Clin Oncol 2020;16:158-64. [Crossref] [PubMed]
- Cavallaro A, Piccolo G, Di Vita M, et al. Managing the incidentally detected gallbladder cancer: algorithms and controversies. Int J Surg 2014;12:S108-19. [Crossref] [PubMed]
- de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999;341:1368-78. [Crossref] [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative man-agement of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [Crossref] [PubMed]
- Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangi-ocarcinoma. Oncologist 2004;9:43-57. [Crossref] [PubMed]
- Ma N, Cheng H, Qin B, et al. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer 2015;15:615. [Crossref] [PubMed]
- Rangarajan K, Simmons G, Manas D, et al. Systemic adjuvant chemotherapy for cholangiocarcinoma surgery: A systematic review and meta-analysis. Eur J Surg Oncol 2020;46:684-93. [Crossref] [PubMed]
- Naveed S, Qari H, Thau CM, et al. Neoadjuvant Chemotherapy for Advanced Gallbladder Cancer: Do We have Enough Evidence? A Systematic Review. Euroa-sian J Hepatogastroenterol 2021;11:87-94. [Crossref] [PubMed]
- Sternby Eilard M, Lundgren L, Cahlin C, et al. Surgical treatment for gallbladder cancer - a systematic literature review. Scand J Gastroenterol 2017;52:505-14. [Crossref] [PubMed]
- Zhao X, Li XY, Ji W. Laparoscopic versus open treatment of gallbladder cancer: A systematic review and meta-analysis. J Minim Access Surg 2018;14:185-91. [Crossref] [PubMed]
- Kellil T, Chaouch MA, Aloui E, et al. Incidence and Preoperative Predictor Factors of Gallbladder Cancer Before Laparoscopic Cholecystectomy: a Systematic Review. J Gastrointest Cancer 2021;52:68-72. [Crossref] [PubMed]
- Dasari BVM, Ionescu MI, Pawlik TM, et al. Outcomes of surgical resection of gallbladder cancer in patients presenting with jaundice: A systematic review and meta-analysis. J Surg Oncol 2018;118:477-85. [Crossref] [PubMed]
- Khan SM, Emile SH, Choudhry MS, et al. Tumor location and concurrent liver resection, impact survival in T2 gallbladder cancer: a meta-analysis of the literature. Updates Surg 2021;73:1717-26. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for sys-tematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Lv TR, Wang JK, Hu HJ, et al. The Significance of Tumor Locations in Patients with Gallbladder Carcinoma After Curative-Intent Resection. J Gastrointest Surg 2023;27:1387-99. [Crossref] [PubMed]
- Yuza K, Sakata J, Hirose Y, et al. Outcome of radical surgery for gallbladder carcinoma according to TNM stage: implications for adjuvant therapeutic strategies. Langenbecks Arch Surg 2021;406:801-11. [Crossref] [PubMed]
- Jeong Y, Park JH, Lee YJ, et al. Postoperative radiotherapy for gallbladder cancer. Anticancer Res 2014;34:5621-9. [PubMed]
- You C, Xie M, Ling M, et al. Residual cancer is a strong predictor of survival in T3 incidental gallbladder cancer. BMC Surg 2022;22:443. [Crossref] [PubMed]
- Navarro J, Kang I, Hwang HK, et al. Glucose to Lymphocyte Ratio as a Prog-nostic Marker in Patients With Resected pT2 Gallbladder Cancer. J Surg Res 2019;240:17-29. [Crossref] [PubMed]
- Choi SB, Han HJ, Kim CY, et al. Surgical outcomes and prognostic factors for T2 gallbladder cancer following surgical resection. J Gastrointest Surg 2010;14:668-78. [Crossref] [PubMed]
- Lim H, Seo DW, Park DH, et al. Prognostic factors in patients with gallbladder cancer after surgical resection: analysis of 279 operated patients. J Clin Gastroen-terol 2013;47:443-8. [Crossref] [PubMed]
- Bergquist JR, Shah HN, Habermann EB, et al. Adjuvant systemic therapy after resection of node positive gallbladder cancer: Time for a well-designed trial? (Re-sults of a US-national retrospective cohort study). Int J Surg 2018;52:171-9. [Crossref] [PubMed]
- Kim Y, Amini N, Wilson A, et al. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann Surg Oncol 2016;23:2998-3008. [Crossref] [PubMed]
- Kwon W, Kim H, Han Y, et al. Role of tumour location and surgical extent on prognosis in T2 gallbladder cancer: an international multicentre study. Br J Surg 2020;107:1334-43. [Crossref] [PubMed]
- Lafaro K, Blakely AM, Melstrom LG, et al. Prognostic impact of tumor location in resected gallbladder cancer: A national cohort analysis. J Surg Oncol 2020;122:1084-93. [Crossref] [PubMed]
- Shindoh J, de Aretxabala X, Aloia TA, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multi-center study. Ann Surg 2015;261:733-9. [Crossref] [PubMed]
- Liu F, Hu HJ, Ma WJ, et al. Prognostic significance of neutrophil-lymphocyte ratio and carbohydrate antigen 19-9 in patients with gallbladder carcinoma. Medi-cine (Baltimore) 2019;98:e14550. [Crossref] [PubMed]
- Xu W, Wu X, Wang X, et al. Prognostic Significance of the Preoperative Lymphocyte to Monocyte Ratio in Patients with Gallbladder Carcinoma. Cancer Manag Res 2020;12:3271-83. [Crossref] [PubMed]
- Shirai Y, Sakata J, Wakai T, et al. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol 2012;10:87. [Crossref] [PubMed]
- Utsumi M, Kitada K, Tokunaga N, et al. A combined prediction model for bil-iary tract cancer using the prognostic nutritional index and pathological findings: a single-center retrospective study. BMC Gastroenterol 2021;21:375. [Crossref] [PubMed]
- Sun L, Ke X, Wang D, et al. Prognostic Value of the Albu-min-to-γ-glutamyltransferase Ratio for Gallbladder Cancer Patients and Establishing a Nomogram for Overall Survival. J Cancer 2021;12:4172-82. [Crossref] [PubMed]
- Xu WY, Zhang HH, Xiong JP, et al. Prognostic significance of the fibrino-gen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol 2018;24:3281-92. [Crossref] [PubMed]
- Blakely AM, Wong P, Chu P, et al. Intraoperative bile spillage is associated with worse survival in gallbladder adenocarcinoma. J Surg Oncol 2019;120:603-10. [Crossref] [PubMed]
- Yao WY, Wu XS, Liu SL, et al. Preoperative lymphocyte to C-reactive protein ratio as a new prognostic indicator in patients with resectable gallbladder cancer. Hepatobiliary Pancreat Dis Int 2022;21:267-72. [Crossref] [PubMed]
- Ethun CG, Le N, Lopez-Aguiar AG, et al. Pathologic and Prognostic Implica-tions of Incidental versus Nonincidental Gallbladder Cancer: A 10-Institution Study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg 2017;83:679-86. [Crossref] [PubMed]
- Kim WJ, Lim TW, Park PJ, et al. Clinicopathological Differences in T2 Gallbladder Cancer According to Tumor Location. Cancer Control 2020;27:1073274820915514. [Crossref] [PubMed]
- Lee W, Jeong CY, Jang JY, et al. Do hepatic-sided tumors require more exten-sive resection than peritoneal-sided tumors in patients with T2 gallbladder cancer? Results of a retrospective multicenter study. Surgery 2017;162:515-24. [Crossref] [PubMed]
- Lee H, Choi DW, Park JY, et al. Surgical Strategy for T2 Gallbladder Cancer According to Tumor Location. Ann Surg Oncol 2015;22:2779-86. [Crossref] [PubMed]
- Yagi H, Shimazu M, Kawachi S, et al. Retrospective analysis of outcome in 63 gallbladder carcinoma patients after radical resection. J Hepatobiliary Pancreat Surg 2006;13:530-6. [Crossref] [PubMed]
- Wang RT, Zhang LQ, Mu YP, et al. Prognostic significance of preoperative platelet count in patients with gallbladder cancer. World J Gastroenterol 2015;21:5303-10. [Crossref] [PubMed]
- Uzun MA, Tilki M, Alkan Kayaoğlu S, et al. Long-term results and prognostic factors after surgical treatment for gallbladder cancer. Turk J Surg 2022;38:334-44. [Crossref] [PubMed]
- Kim M, Kim H, Han Y, et al. Prognostic Value of Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen 19-9 (CA 19-9) in Gallbladder Cancer; 65 IU/mL of CA 19-9 Is the New Cut-Off Value for Prognosis. Cancers (Basel) 2021;13:1089. [Crossref] [PubMed]
- Saluja SS, Nekarakanti PK, Mishra PK, et al. Prospective Randomized Con-trolled Trial Comparing Adjuvant Chemotherapy vs. No Chemotherapy for Patients with Carcinoma of Gallbladder Undergoing Curative Resection. J Gastrointest Surg 2022;26:398-407. [Crossref] [PubMed]
- Feroz Z, Gautam P, Tiwari S, et al. Survival analysis and prognostic factors of the carcinoma of gallbladder. World J Surg Oncol 2022;20:403. [Crossref] [PubMed]
- Gupta S, Prakash P, Kumar V, et al. Radical surgery for de novo gallbladder carcinoma-Single-center analysis of prognostic factors and survival outcomes from an endemic region. J Surg Oncol 2022;125:631-41. [Crossref] [PubMed]
- Cui X, Zhu S, Tao Z, et al. Long-term outcomes and prognostic markers in gallbladder cancer. Medicine (Baltimore) 2018;97:e11396. [Crossref] [PubMed]
- Deng Y, Zhang F, Yu X, et al. Prognostic Value Of Preoperative Systemic In-flammatory Biomarkers In Patients With Gallbladder Cancer And The Establishment Of A Nomogram. Cancer Manag Res 2019;11:9025-35. [Crossref] [PubMed]
- Zhu S, Yang J, Cui X, et al. Preoperative platelet-to-lymphocyte ratio and neu-trophil-to-lymphocyte ratio as predictors of clinical outcome in patients with gallbladder cancer. Sci Rep 2019;9:1823. [Crossref] [PubMed]
- de Aretxabala X, Roa I, Burgos L, et al. Gallbladder cancer: an analysis of a series of 139 patients with invasion restricted to the subserosal layer. J Gastrointest Surg 2006;10:186-92. [Crossref] [PubMed]
- Cho JK, Lee W, Jang JY, et al. Validation of the oncologic effect of hepatic resection for T2 gallbladder cancer: a retrospective study. World J Surg Oncol 2019;17:8. [Crossref] [PubMed]
- Dai H, Xu J. Preoperative geriatric nutritional risk index is an independent prognostic factor for postoperative survival after gallbladder cancer radical surgery. BMC Surg 2022;22:133. [Crossref] [PubMed]
- Wang L, Dong P, Zhang Y, et al. Prognostic validation of the updated 8th edi-tion Tumor-Node-Metastasis classification by the Union for International Cancer Control: Survival analyses of 307 patients with surgically treated gallbladder car-cinoma. Oncol Lett 2018;16:4427-33. [PubMed]
- Koh CY, Demirjian AN, Chen WP, et al. Validation of revised American Joint Committee on Cancer staging for gallbladder cancer based on a single institution experience. Am Surg 2013;79:1045-9. [Crossref] [PubMed]
- Chen H, Huang Z, Sun B, et al. The predictive value of systemic immune in-flammation index for postoperative survival of gallbladder carcinoma patients. J Surg Oncol 2021;124:59-66. [Crossref] [PubMed]
- Zhang W, Huang Z, Wang WE, et al. Survival Benefits of Simple Versus Ex-tended Cholecystectomy and Lymphadenectomy for Patients With T2 Gallbladder Cancer: A Propensity-Matched Population-Based Study (2010 to 2015). Front Oncol 2021;11:705299. [Crossref] [PubMed]
- Fan DX, Xu RW, Li YC, et al. Impact of the number of examined lymph nodes on outcomes in patients with lymph node-negative gallbladder carcinoma. World J Gastroenterol 2018;24:2886-92. [Crossref] [PubMed]
- Kim SH, Chong JU, Lim JH, et al. Optimal assessment of lymph node status in gallbladder cancer. Eur J Surg Oncol 2016;42:205-10. [Crossref] [PubMed]
- Deng Y, Pang Q, Bi JB, et al. A promising prediction model for survival in gallbladder carcinoma patients: pretreatment prognostic nutrient index. Tumour Biol 2016; Epub ahead of print. [Crossref] [PubMed]
- Bao Y, Yang J, Duan Y, et al. The C-reactive protein to albumin ratio is an ex-cellent prognostic predictor for gallbladder cancer. Biosci Trends 2021;14:428-35. [Crossref] [PubMed]
- Choudhary S, Asthana AK. Impact of Adjuvant Therapy on Survival in Cura-tively Resected Gallbladder Carcinoma. J Clin Diagn Res 2015;9:XC01-4.
- Zou RQ, Hu HJ, Lv TR, et al. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma of the gallbladder. Front Oncol 2022;12:1009673. [Crossref] [PubMed]
- Kang MJ, Song Y, Jang JY, et al. Role of radical surgery in patients with stage IV gallbladder cancer. HPB (Oxford) 2012;14:805-11. [Crossref] [PubMed]
- Go SI, Kim YS, Hwang IG, et al. Is There a Role for Adjuvant Therapy in R0 Resected Gallbladder Cancer?: A Propensity Score-Matched Analysis. Cancer Res Treat 2016;48:1274-85. [Crossref] [PubMed]
- Tao Z, Li SX, Cui X, et al. The prognostic value of preoperative inflammatory indexes in gallbladder carcinoma with hepatic involvement. Cancer Biomark 2018;22:551-7. [Crossref] [PubMed]
- Pang Q, Zhang LQ, Wang RT, et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. World J Gastroenterol 2015;21:6675-83. [Crossref] [PubMed]
- Nakakubo Y, Miyamoto M, Cho Y, et al. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer 2003;89:1736-42. [Crossref] [PubMed]
- Rogula B, Lozano-Ortega G, Johnston KM. A Method for Reconstructing Indi-vidual Patient Data From Kaplan-Meier Survival Curves That Incorporate Marked Censoring Times. MDM Policy Pract 2022;7:23814683221077643. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003;22:2693-710. [Crossref] [PubMed]
- Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123-32. [Crossref] [PubMed]
- Hamdani NH, Qadri SK, Aggarwalla R, et al. Clinicopathological study of gall bladder carcinoma with special reference to gallstones: our 8-year experience from eastern India. Asian Pac J Cancer Prev 2012;13:5613-7. [Crossref] [PubMed]
- Grobmyer SR, Lieberman MD, Daly JM. Gallbladder cancer in the twentieth century: single institution’s experience. World J Surg 2004;28:47-9. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tu-mour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665-74. [Crossref] [PubMed]
- Pagès F, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010;29:1093-102. [Crossref] [PubMed]
- Wang YF, Feng FL, Zhao XH, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol 2014;20:4085-92. [Crossref] [PubMed]
- Dowaki S, Kijima H, Kashiwagi H, et al. CEA immunohistochemical localiza-tion is correlated with growth and metastasis of human gallbladder carcinoma. Int J Oncol 2000;16:49-53. [PubMed]
- Sachan A, Saluja SS, Nekarakanti PK, et al. Raised CA19-9 and CEA have prognostic relevance in gallbladder carcinoma. BMC Cancer 2020;20:826. [Crossref] [PubMed]
- Xu WY, Zhang HH, Yang XB, et al. Prognostic significance of combined pre-operative fibrinogen and CA199 in gallbladder cancer patients. World J Gastroen-terol 2018;24:1451-63. [Crossref] [PubMed]
- Liu GJ, Li XH, Chen YX, et al. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J Gastroenterol 2013;19:5150-8. [Crossref] [PubMed]
- Kang H, Choi YS, Suh SW, et al. Prognostic Significance of Tumor Location in T2 Gallbladder Cancer: A Systematic Review and Meta-Analysis. J Clin Med 2021;10:3317. [Crossref] [PubMed]
- Hawkins WG, DeMatteo RP, Jarnagin WR, et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol 2004;11:310-5. [Crossref] [PubMed]
- Vega EA, Vinuela E, Okuno M, et al. Incidental versus non-incidental gallbladder cancer: index cholecystectomy before oncologic re-resection negatively impacts survival in T2b tumors. HPB (Oxford) 2019;21:1046-56. [Crossref] [PubMed]
- Buitrago-Molina LE, Dywicki J, Noyan F, et al. Splenectomy Prior to Experi-mental Induction of Autoimmune Hepatitis Promotes More Severe Hepatic In-flammation, Production of IL-17 and Apoptosis. Biomedicines 2021;9:58. [Crossref] [PubMed]
- Wang Y, Wen N, Wang S, et al. Chemotherapy and targeted therapy for ad-vanced biliary tract cancers: an umbrella review. BMC Cancer 2023;23:378. [Crossref] [PubMed]
- Sinha S, Engineer R, Ostwal V, et al. Radiotherapy for locally advanced unre-sectable gallbladder cancer - A way forward: Comparative study of chemotherapy versus chemoradiotherapy. J Cancer Res Ther 2022;18:147-51. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of bil-iary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Okumura K, Gogna S, Gachabayov M, et al. Gallbladder cancer: Historical treatment and new management options. World J Gastrointest Oncol 2021;13:1317-35. [Crossref] [PubMed]
- Hoehn RS, Wima K, Ertel AE, et al. Adjuvant Therapy for Gallbladder Cancer: an Analysis of the National Cancer Data Base. J Gastrointest Surg 2015;19:1794-801. [Crossref] [PubMed]






