Liver transplantation for hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) or hepatoma is the sixth most common cancer worldwide and the third most common cause of death from cancer. In the United States, the incidence is rising and is expected to continue rising over the next two decades. It is the most common primary tumor of the liver accounting for 90% of all primary liver tumors. Mean survival is estimated to be 6 to 20 months without intervention (1). Unfortunately, platin and adriamycin based chemotherapic and radiation therapies do not offer substantial survival benefits. Recently, sorafenib has been approved by the US FDA for the treatment of unresectable HCC (2,3). This new therapeutic option may open more doors that include liver transplantation. Furthermore there is increasing evidence that sirolimus may further improve post transplant cancer disease free survival (4). Over the last thirty years, the treatment of this cancer has changed greatly. Advances in surgical technique and immunosuppression regimens have made liver transplantation a feasible alternative to many patients with HCC.
Etiology
The most common cause of hepatoma is chronic hepatitis virus infection. The prevalence of HCC parallels that of viral hepatitis across the globe. Whereas chronic hepatitis B infection is the most common cause of HCC worldwide and in most African and Asian countries, chronic hepatitis C virus is the leading cause in southern European countries and North America (Figure 1A,B).
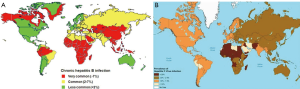
Chronic hepatitis B infection is well defined as an etiology for hepatoma. Three quarters of the cases of HCC occur in Asian countries where there is a high prevalence of chronic hepatitis B infection. The mechanism remains unclear, but some have postulated that the DNA viral replication plays a role. What is known is that there is an increased relative risk (223×) among carriers to developing this cancer (5,6). Furthermore, there is increasing evidence that active viral replication in hepatitis B patients increases this risk of hepatocellular cancer in those who are chronically infected (7,8). Lastly, there is a positive correlation between specific viral variants, (namely genotype C, precore, basal core and pre-S deletion mutants) and cancer development (9).
In contrast, chronic hepatitis C infection is a more common etiology in Europe and North America. It is reasoned that this could be related to a hepatitis C viral epidemic thirty years ago in the developed world. Nevertheless, it is known that about four million Americans have chronic hepatits C and roughly one third will progress to chronic liver disease, many of these patients will go on to develop cancer. This pattern of hepatitis to cirrhosis to carcinoma readily distinguishes hepatitis C patients from their hepatitis B counterparts and carries implications for their treatment and outcomes. Interestingly, also in contrast to hepatitis B patients, the distribution of chronic hepatitis C patients varies between regions and ethnic groups within countries where the disease is endemic, suggesting that there is a social or behavioural component to transmission (10).
In chronic hepatitis patients, regardless of whether the causitive virus is B or C, a number of independent risk factors have been identified. Male gender is one - in high risk countries, the ratio is 3:1 (male:female). Advanced age is another, particularly in areas where hepatitis C virus is endemic (11). In hepatitis B endemic areas, incidence rates increase after age 20. Obesity, family history, diabetes and alcoholism increase the cancer risk in chronically infected patients (6). It is known too that liver disease progresses faster in patients with HIV coinfection (12,13). Additionally, hepatitis B - hepatitis C coinfection has a synergistic effect in the development of carcinoma (14).
Aflatoxin is produced from fungi and is a common contaminant in corn, peanuts and soy beans in Asian countries such as China and Taiwan. It is a known carcinogen in the development of hepatocellular cancer (15,16). Another less common etiology of this cancer is hereditary hemochromatosis. Its mechanism is believed to similar to hepatitis C, in that persistent inflammation leads to fibrosis, cirrhosis and eventual cancer (17,18). Other less common causes of HCC include ethanol ingestion, primary biliary cirrhosis, alpha-a antitrypsin deficiency, hypercitrullinemia, porphyrias, hereditary tyrosemia, Wilson’s disease and carcinogenic agents such as thorotrast, polyvinyl chloride and carbon chloride (19).
Pathology
The gross pathologic appearance of HCC varies depending on the presence of cirrhosis. Multinodular lesions in noncirrhotic livers typically reflects intrahepatic metastases. Whereas, cirrhotic livers are usually representative of multicentric HCC. Multicentric tumors are common in patients with hepatitis C and tend to grow in the most damaged segments of the liver. The color may be green due to bile production, yellow due to fatty infiltrates, tan-brown, or grey-white.
Hematologic spread most often affects the lungs (48%), followed by the adrenal glands (8.3%), bone (5.6%), gastrointestinal tract (4.7%), bladder (3.5%) and pancreas (3%) (20). Up to one quarter of tumors present with lymph node metastases, typically to the hilar, peripancreatic, perigastric and periaortic nodes.
The histological appearance of this tumor is highly variable. The most common form is the trabecular pattern which encompasses the pseudoglandular, pseudofollicular and mixed trabecular-acinar types. The pseudo-glandular and acinar pattern is characterized by dilated bile cannuiculus-like structures, often filled with bile (1). Other patterns that describe the tumor include solid, compact, scirrhous, clear cell, giant cell, pseudocapsular, and sarcomatous. The compact variant is sinusoid-like blood spaces that are slit-like. The scirrhous type is distinguished by marked fibrosis (9).
On cytology, tumor cells of HCC may show fatty change, Mallory bodies, globular hyaline bodies, pale bodies, pleomorphic cells and sarcomatous changes. HCC often contains more than one cytologic variant within the same tumor. The production of bile, the expression of alpha fetoprotein, the canalicular expression pattern of biliary glycoprotein 1 and CD10, lack of reticulin, and the presence of albumin mRNA are all distinguishing features of HCC that separate it from other solid tumors in the liver.
Molecular markers
Alpha fetoprotein (AFP) is highly diagnostic for this tumor. It is present in large quantities during fetal development but decreases rapidly after birth. Normal adult level is typically less than 10 ng. Typically, elevated levels of AFP greater than 400 ng/mL are considered diagnostic. This marker may return to normal after resection and is useful as a marker for tumor recurrence. Mild elevations in AFP may be found in acute viral hepatitis, chronic liver disease, and some metastatic cancers. Fulminant HBV, teratocarcinomas, yolk sac tumors and metastatic tumors from the stomach or pancreas can also produce markedly elevated levels. As a diagnostic tool, AFP is most helpful in concordance with hepatic imaging confirming the presence of tumor.
Two other tumor markers, des-gamma-carboxyprothrombin (DCP) and alpha L-fructosidase (AFP-L3), are also possible markers for HCC. DCP is an abnormal prothrombin protein that is increased HCC patients. It is highly specific for the disease and may also be a predictor of progrnosis. Current Asian consensus guidelins advocate for routine use of AFP and DCP to increase sensitivity in detection of HCC. AFP-L3 is a fucosylated variant of AFP that can help to differentiate an increase in AFP due to HCC versus benign liver disease. Recently Mao et al. described using GP-73 as an adjuct to AFP to increase sensitivity and specificity (21,22).
Radiographic imaging
The 2010 practice guideline recommendations of the American Association for the Study of Liver Diseases state that HCC can be diagnosed on the basis of radiologic findings without biopsy. There are two criteria for diagnosis: arterial enhancement of a nodule and the presence of washout on portal venous or delayed imaging. For this purpose, contrast-enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) is the best studies.
Contrast-enhanced ultrasound does have potential utility but its use is limited by the lack of availability of the necessary contrast agents in many countries, including the United States, and the inherent limitations of ultrasound, such as operator-dependent variability and patient habitus. Regardless of imaging technique employed, patients with hepatocellular cancer patients should undergo a metastatic workup. For this purpose, a CT or MRI of the abdomen and pelvis, a CT of the chest and bone scintigraphy should be obtained.
Staging
There are a number of staging systems that assess liver function and tumor burden based on radiological or pathological criteria. Although none have been universally accepted, four have gained widespread acceptance. These will be discussed first, followed by the others. Of these classifications, only two, BCLC and GRETCH, consider performance status. CUPI is the only one to assess symptomatic disease.
The TNM classification was developed by International Union Against Cancer and American Joint Committee on Cancer and has been validated for good discrimination between stages for patients undergoing hepatic resection. It is based on tumor size, the number of tumors and extent of disease, including vascular invasion. This staging system was most recently revised in 2010 to accommodate for prognostic implications of multiple tumors and vascular involvement. The TNM classification has been validated in large cohort trials is considered the most accurate to define post-transplant outcomes (2). Still, it has been criticized for its complexity and its failure to adequately stratify patients with cirrhosis and large tumors.
In this group of patients, the Okunda classification is considered more useful as a prognostic indicator. This staging system was developed in 1985 and, as it does not stratify patients who are not candidates for resection, is a purely clinical scoring system. The Okunda classification is based on tumor size and the severity of cirrhosis. It is limited by the absence of assessment of tumor burden.
The Cancer of the Liver Italian Program (CLIP) was introduced in 1998 and validated as a prognostic inidcator in 2000. It includes Child-Pugh, tumor morphology and extent, presence of portal vein thrombosis and AFP level. Several studies suggest that CLIP may be better at predicting survival than either TNM or Okunda classifications, particularly in patients undergoing adjuvant therapy (23).
The Barcelona Clinic Liver Cancer (BCLC) system includes performance status, presence of multifocal tumor lesions, vascular invasion, extrahepatic spread, Child-Pugh stage, portal hypertension. This classification is criticized for being algorithmic rather than being patient-centered. However, recent studies have deemed this the best prognositc system (23). The American Heptopancreaticobiliary Association consensus statement recommends the BCLC scheme for patients with advanced cancer who are not candidates for surgery and the TNM staging for candidates who meet criteria for liver resection (24).
The Liver Cancer Study Group of Japan (LCSGJ) utilizes revised TNM staging for clinical and pathologic staging of primary liver cancer. It includes twelve classifications and has been criticized for its complexity and lack of prognostic correlation.
The Japan Integrated Staging Score (JIS) was developed in 2003 and combines TNM stage and Child-Pugh Stage into a score of 0 to 5. It has yet to be validated in populations outside of Japan. However, it has been compared to the CLIP and BCLC systems and found to be a superior prognostic determinant {Kudo, 2004 #3950}. It remains the most popular staging system that country.
The Chinese University Prognostic Index includes nineteen variables and is proven useful in determining prognosis in Southeast Asian populations with HBV-HCC predominance. The Tokyo score developed with a cohort of Japanese patients with early stage disease who were treated with percutaneous ablation and was validated with a cohort undergoing resection surgery. Lastly the Taipei Integrated scoring system uses total tumor volume to assess tumor burden. None of these has been validated or widely used outside of the populations in which and for which they were developed.
Evolution of transolantation for HCC
The first liver transplant performed in humans was done by Dr. Thomas Starzl in 1963. However, the procedure did not gain widespread acceptance until the 1980s when cyclosprine started being used as an immunosuppressive agent. The finding that small, incidentally found tumors in explanted livers did not affect survival introduced the idea of liver transplantation as a treatment for HCC. Still, the use of this modality as a treatment for HCC remained limited by high recurrence rates and low 5-year survival.
However, the advent of the 1990s brought evidence that hepatic transplant could be done safely with good outcomes. In 1991, Dr. Iwatsuki et al. published data from 105 patients with heptoma who underwent liver transplantation. 35% of these patients had portal invasion, and 75% had multinodular tumors. The team reported 36% 5-year survival. {Iwatsuki, 1991 #3963} While still poor, these numbers were more satisfactory than previously reported. Two years later, Bismuth and colleagues, reported better outcomes (49% 3-yr survival) in patients with up to three tumors, each less than 3 cm. The group demonstrated better disease free survival rates after liver transplantation compared with hepatic resection (25).
In 1996, in a landmark paper published in the New England Journal of Medicine, Dr. Mazzafero published results demonstrating 74% 4 year survival after liver transplantation in patients with solitary lesions less than 5 cm in diameter or up to 3 lesions each less than 3 cm in diameter. This has been designated the Milan criteria (26). Three years later the Bismuth group published new data suggesting similar survival rates in patients with tumors less than 3 cm (27). The “Milan Criteria” quickly became the standard. Currently, HCC is the primary indication for liver transplant for 25% of all cases in Europe and 35% of all cases in the United States.
Expanding criteria for liver transplantation
There is a need to optimize benefits given the limited number of available organs. This has lead to the development of stringent criteria for transplantation. Traditionally, most centers employ the Milan criteria. Most notably, the 2010 International Consensus for Transplantation for HCC advocates the use of Milan criteria as the benchmark for selection. But, there is considerable interest in expanding these criteria and certain some centers have shown progress in this area. At the University of California San Francisco, Dr. Yao et al. have demonstrated that patients with a single lesions less than 6.5 cm, or up to three lesions each less than 4 cm with a cumulative diameter less than 8 cm have surgical outcomes similar to those transplanted under Milan criteria (28,29).
There is promising new data which suggests that tumor histology may be more important than tumor burden in determining post-transplantat outcomes. In 2004, Dr. Cillo and his colleagues in Italy reported a retrospective analysis which showed that patients with well to moderate grade HCC had acceptable outcomes after transplantation regardless of tumor burden. Thirteen patients in his cohort did not meet Milan criteria (30). More recently, Dr. DuBay at the University of Toronto reported in 2011 that transplantation in patients with advanced moderate to well differentiated adenocarcinoma can be performed safely. The group reported 5-year survival of 70% and a 5-year disease free survival of 66% which was comparable to those who met Milan criteria in their cohort (31).
Loco-regional therapies
In order to maximize the benefit of transplantation, the course of the disease needs to be arrested while awaiting a suitable graft for transplantation, loco-regional treatment is able to accomplish this goal in most circumstances (32-42). Given the current waiting times in the major cities in the US, most programs have adopted the international consensus report that recommends bridging therapies for patients with T2 disease (solitary tumor 2-5 cm, or two to three lesions 2-3 cm) but not for T1 lesions (solitary tumor without vascular invasion). Transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) are the two modalities most widely employed.
It is now known that the majority of the blood supply to hepatic tumors is derived from the hepatic artery. This fact, combined with advances in technology, has enabled targeted chemotherapeutic intervention for hepatocellular cancers, otherwise known as transarterial chemoembolization or TACE. During TACE procedures, chemotherapeutic agents, such as doxirubicin, cisplatin and mitomycin combinations are injected into the artery supplying the tumor usually with lipiodol or a procoagulant. Lipiodol is an agent that promotes tumor retention of chemotherapy medications (34,37,40,42-45). Similarly, drug-eluting microspheric beads have shown promise as a treatment, possibly with less toxicity (46). Contraindications to this treatment include the absence of hepatopedal blood flow, encephalopathy and biliary obstruction.
Radiofrequency ablation uses high frequency alternating currents from an electrode inserted into the lesion. Ions within the tissue attempt to follow the change in directions of the charge resulting in friction and heat. As the temperature rises above sixty degrees celcius tumor necrosis occurs This method is best utilized in patients with solitary tumors less than four centimeters (32,47-51).
Alternative treatment options
The Sorafenib in Advanced HCC (SHARP) trial demonstrated a modest, statistically significant three-month survival benefit for this medication compared to placebo. It should be noted that it is a toxic drug associated with increased risk of bleeding, poor wound healing, diarrhea and hepatic decompensation. Furthermore, the SHARP trial was limited to patients with Child’s A cirrhosis, making it of limited utility in the general patient population. The median survival in the study group was 10.7 months (33,52-55). Currently, we are awaiting results of phase II trial combining sorafenib and doxorubicin. Still, it is unlikely that medical treatment will offer comparable results to interventional or surgical procedures in the near future.
Graft selection
The critical issue for all patients awaiting liver transplantation is the availability of transplantable grafts. Time is a major determinate of overall survival if one assesses intent to transplant analysis (56-60). The biology of the tumor can impact the time a center is willing to wait for a graft. If there is evidence that the tumor has an aggressive biological behavior, it may be wise to wait (3-6 months) and determine the exact nature of the tumor while tumors that do not demonstrate aggressive behavior should be transplanted as soon as a graft is available. This key clinical difference is very difficult to determine at times (61-64). Living donation is an avenue that is perfect for transplantation in patients with HCC as the time function is eliminated and the transplant can be planned at a time that is optimal in terms of assessing the biological nature of the tumor and minimizes tumor recurrence (65-71). The temptation is to transplant as soon as the donor is worked up, but this may lead to higher recurrence rates. The waiting time allows for self-selection of tumors with favorable tumor biology. It is, at times, difficult for the team to wait once a suitable donor is identified.
Immunosuppression
There is growing evidence that immunosuppressive agents may determine the risk of recurrence after transplantation. Sirolimus is a bacterial macrolide with immunosuppressive and antineoplastic properties. The mechanism of action appears to work via inhibition of IL-2 mediated lymphocyte proliferation. In laboratory studies, this results in decreased metastatic tumor growth and decreased angiogenesis in the liver. Several studies have demonstrated that a post-transplant regimen of sirolimus within a steroid free protocol and a low tacrolimus target is associated with a decreased risk of tumor recurrence without significant risk of infection or hepatic artery thrombosis (4,72-75).
Conclusions
Transplantation as treatment for HCC has enjoyed increasing attention as improvements in surgical technique, immunosuppression and patient selection have lead to increased postoperative survival. Patients that have HCC within the Milan criteria should be treated as any other transplant patient unless there is evidence that the biological nature of the tumor is aggressive. For patients who present with tumors outside of Milan criteria, it is more important to mandate a 3-6 months waiting period to assess the biological nature of the tumor. In all patients during the period of waiting loco-regional therapy should be applied to the tumor. In those patients outside of Milan criteria, the addition of sorafenib should be considered. Similarly, in those patients outside of Milan criteria, a steroid free immunosuppression regimen starting off with a calcineurin inhibitor that is weaned to off with sirolimus started as maintainance immunosuppression around 3 months appears to offer the best chance of long term survival. The role of sorafenib post transplant has not yet been established, but may have a role for those patients at high risk of recurrence.
Acknowledgements
This study was supported by China Medical Board Grant.
Disclosure: The authors declare no conflict of interest.
References
- Society AC. What are the key statistics about liver cancer. 2012 [cited 2012 October 21, 2012]; Available online: http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-what-is-key-statistics
- Ranieri G, Gadaleta-Caldarola G, Goffredo V, et al. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem 2012;19:938-44.
- Kudo M. Targeted therapy for liver cancer: updated review in 2012. Curr Cancer Drug Targets 2012. [Epub ahead of print].
- Kneteman NM, Oberholzer J, Al Saghier M, et al. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl 2004;10:1301-11.
- Chakrabarty SP, Murray JM. Modelling hepatitis C virus infection and the development of hepatocellular carcinoma. J Theor Biol 2012;305:24-9.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1.
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74.
- Shiha G, Sarin SK, Ibrahim AE, et al. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009;3:323-33.
- Röcken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Dig Dis 2001;19:269-78.
- NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements 2002;19:1-46.
- Mair RD, Valenzuela A, Ha NB, et al. Incidence of Hepatocellular Carcinoma Among US Patients With Cirrhosis of Viral or Nonviral Etiologies. Clin Gastroenterol Hepatol 2012;10:1412-7.
- Nunnari G, Berretta M, Pinzone MR, et al. Hepatocellular carcinoma in HIV positive patients. Eur Rev Med Pharmacol Sci 2012;16:1257-70.
- Puoti M, Rossotti R, Garlaschelli A, et al. Hepatocellular carcinoma in HIV hepatitis C virus. Curr Opin HIV AIDS 2011;6:534-8.
- Riaz M, Idrees M, Kanwal H, et al. An overview of triple infection with hepatitis B, C and D viruses. Virol J 2011;8:368.
- Lereau M, Gouas D, Villar S, et al. Interactions between hepatitis B virus and aflatoxin B(1): effects on p53 induction in HepaRG cells. J Gen Virol 2012;93:640-50.
- Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol 2012;13:218-28.
- Villanueva A, Newell P, Hoshida Y. Inherited hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2010;24:725-34.
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004;127:S79-86.
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50.
- Si MS, Amersi F, Golish SR, et al. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg 2003;69:879-85.
- Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010;59:1687-93.
- Mao YL, Yang HY, Xu HF, et al. Significance of Golgi glycoprotein 73, a new tumor marker in diagnosis of hepatocellular carcinoma: a primary study. Zhonghua Yi Xue Za Zhi 2008;88:948-51.
- Olthoff KM, Forner A, Hübscher S, et al. What is the best staging system for hepatocellular carcinoma in the setting of liver transplantation? Liver Transpl 2011;17:S26-33.
- Helton WS, Strasberg SM. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: rationale and overview of the conference. HPB (Oxford) 2003;5:238-42.
- Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993;218:145-51.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9.
- Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis 1999;19:311-22.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765-74.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403.
- Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg 2004;239:150-9.
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72.
- Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci 2012;57:3026-31.
- Yamashita T, Kaneko S. Treatment strategies for hepatocellular carcinoma in Japan. Hepatol Res 2012. [Epub ahead of print].
- Takaki S, Sakaguchi H, Anai H, et al. Long-term outcome of transcatheter subsegmental and segmental arterial chemoemobolization using lipiodol for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2012;35:544-54.
- Shi XJ, Jin X, Wang MQ, et al. Effect of resection following downstaging of unresectable hepatocelluar carcinoma by transcatheter arterial chemoembolization. Chin Med J (Engl) 2012;125:197-202.
- Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73.
- Rammohan A, Sathyanesan J, Ramaswami S, et al. Embolization of liver tumors: Past, present and future. World J Radiol 2012;4:405-12.
- Qu XD, Chen CS, Wang JH, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer 2012;12:263.
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43-58.
- Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol 2012;39:503-9.
- Kwan SW, Fidelman N, Ma E, et al. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl 2012;18:727-36.
- Eswaran SL, Pierce K, Weaver F, et al. Transarterial chemoembolization for HCC in patients with extensive liver transplantation waiting times. Angiology 2012;63:206-12.
- Yamanaka K, Hatano E, Kitamura K, et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol 2012;47:343-6.
- Lencioni R. Management of hepatocellular carcinoma with transarterial chemoembolization in the era of systemic targeted therapy. Crit Rev Oncol Hematol 2012;83:216-24.
- Bargellini I, Sacco R, Bozzi E, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol 2012;81:1173-8.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 2012;56:1330-5.
- Yu CY, Ou HY, Huang TL, et al. Hepatocellular carcinoma downstaging in liver transplantation. Transplant Proc 2012;44:412-4.
- Sourianarayanane A, El-Gazzaz G, Sanabria JR, et al. Loco-regional therapy in patients with Milan Criteria-compliant hepatocellular carcinoma and short waitlist time to transplant: an outcome analysis. HPB (Oxford) 2012;14:325-32.
- Sheiman RG, Mullan C, Ahmed M. In vivo determination of a modified heat capacity of small hepatocellular carcinomas prior to radiofrequency ablation: correlation with adjacent vasculature and tumour recurrence. Int J Hyperthermia 2012;28:122-31.
- N’Kontchou G, Aout M, Laurent A, et al. Survival after radiofrequency ablation and salvage transplantation in patients with hepatocellular carcinoma and Child-Pugh A cirrhosis. J Hepatol 2012;56:160-6.
- Meza-Junco J, Montano-Loza AJ, Liu DM, et al. Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev 2012;38:54-62.
- Raoul JL, Bruix J, Greten TF, et al. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol 2012;56:1080-8.
- Ang C, O’Reilly EM, Abou-Alfa GK. Targeted agents and systemic therapy in hepatocellular carcinoma. Recent Results Cancer Res 2013;190:225-46.
- Zhang X, Yang XR, Huang XW, et al. Sorafenib in treatment of patients with advanced hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int 2012;11:458-66.
- Woo HY, Heo J. Sorafenib in liver cancer. Expert Opin Pharmacother 2012;13:1059-67.
- Avolio AW, Siciliano M, Barone M, et al. Model for end-stage liver disease dynamic stratification of survival benefit. Transplant Proc 2012;44:1851-6.
- Lai JC, Feng S, Roberts JP. An examination of liver offers to candidates on the liver transplant wait-list. Gastroenterology 2012;143:1261-5.
- Maggs JR, Suddle AR, Aluvihare V, et al. Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:1113-34.
- Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps. Liver Transpl 2011;17:1005-12.
- Gordon Burroughs S, Busuttil RW. Optimal utilization of extended hepatic grafts. Surg Today 2009;39:746-51.
- Yaprak O, Akyildiz M, Dayangac M, et al. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2012;11:256-61.
- Singal AG, Chan V, Getachew Y, et al. Predictors of liver transplant eligibility for patients with hepatocellular carcinoma in a safety net hospital. Dig Dis Sci 2012;57:580-6.
- Zarrinpar A, Kaldas F, Busuttil RW. Liver transplantation for hepatocellular carcinoma: an update. Hepatobiliary Pancreat Dis Int 2011;10:234-42.
- Vivarelli M, Risaliti A. Liver transplantation for hepatocellular carcinoma on cirrhosis: strategies to avoid tumor recurrence. World J Gastroenterol 2011;17:4741-6.
- Chok KS, Chan SC, Cheung TT, et al. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg 2011;35:2058-62.
- Kulik LM, Fisher RA, Rodrigo DR, et al. Outcomes of Living and Deceased Donor Liver Transplant Recipients With Hepatocellular Carcinoma: Results of the A2ALL Cohort(†). Am J Transplant 2012;12:2997-3007.
- Liang W, Wu L, Ling X, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: A meta-analysis. Liver Transpl 2012;18:1226-36.
- Moon JI, Kwon CH, Joh JW, et al. Primary versus salvage living donor liver transplantation for patients with hepatocellular carcinoma: impact of microvascular invasion on survival. Transplant Proc 2012;44:487-93.
- Sandhu L, Sandroussi C, Guba M, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl 2012;18:315-22.
- Earl TM, Chapman WC. Transplantation for hepatocellular carcinoma: the north american experience. Recent Results Cancer Res 2013;190:145-64.
- Lee SG, Moon DB. Living donor liver transplantation for hepatocellular carcinoma. Recent Results Cancer Res 2013;190:165-79.
- Kawahara T, Asthana S, Kneteman NM. m-TOR inhibitors: what role in liver transplantation? J Hepatol 2011;55:1441-51.
- Schnitzbauer AA, Zuelke C, Graeb C, et al. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer 2010;10:190.
- Toso C, Merani S, Bigam DL, et al. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010;51:1237-43.
- Toso C, Meeberg GA, Bigam DL, et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation 2007;83:1162-8.


