
Splenic hypertrophy predicts liver-specific complications in patients undergoing major liver resection for colorectal liver metastases, after preoperative chemotherapy
Highlight box
Key findings
• Splenic hypertrophy (SH), following preoperative chemotherapy, is associated with postoperative complications after major liver resection for colorectal liver metastases (CRLM). A threshold of 8.6% SH was identified as predictive of postoperative liver-specific complications (LSC).
• The predictive ability of SH was particularly strong in overweight (body mass index >25 kg/m2) patients. Here, cut-off values were identified for LSC, major complications (Clavien-Dindo ≥3) and a Comprehensive Complication Index >50.
What is known and what is new?
• Despite the advantages of preoperative chemotherapy in CRLM patients, a well-known side-effect is non-alcoholic fatty liver disease (yellow liver) and sinusoidal obstruction syndrome (blue liver). These are associated with increased postoperative morbidity and can cause SH, as a result of portal hypertension.
• To our knowledge, this is the first study to examine post-chemotherapy SH as a predictor of morbidity after major liver resection for CRLM, in the modern era, and with a sizeable cohort. We have defined a cut-off value for the prediction of LSC and have also demonstrated an increased effect in overweight patients.
What is the implication, and what should change now?
• Patients exposed to systemic treatment before liver resection are at increased risk of postoperative morbidity, because of chemotherapy-associated liver injury. As these patients routinely undergo imaging to assess tumor response, splenic volume measurements could be carried out to identify those at risk of postoperative complications.
• Overweight patients could particularly benefit from this screening process.
Introduction
Background
Colorectal liver metastases (CRLM) are detected in up to 75% of colorectal cancer patients and are the predominant limiting factor of survival (1). Radical surgery is the gold standard, if complete resection is possible (2), yet only 15–20% of cases are initially eligible for curative surgery (3). Preoperative chemotherapy may decrease metastatic load, enabling approximately 20% more patients with initially irresectable CRLM to be considered for surgery (4,5). Furthermore, chemotherapy can improve survival rates and significantly delay recurrence (6). However, cytotoxic agents can also cause chemotherapy-associated liver injury (CALI), characterized by distinctive macroscopic alterations in the liver, often described as “blue-” or “yellow liver” (2). These hepatic alterations have been linked to an elevated risk of postoperative complications, particularly following major hepatic resection (6-8).
Rationale and knowledge gap
In 2004, Rubbia-Brandt et al. detected specific morphological liver damage in patients treated with oxaliplatin, which is referred to as sinusoidal obstruction syndrome (SOS) (9). This is characterized by sinusoidal dilatation, fibrosis, and nodular regenerative hyperplasia, affecting roughly 78% of oxaliplatin-treated patients and may increase postoperative complication and morbidity rates up to 38% (2,4,9,10). The preoperative diagnosis of SOS remains challenging, as liver biopsies are not routinely taken before resection, and radiological diagnosis is challenging (11).
Studies have linked SOS and consequent portal hypertension with splenomegaly (12-14). Splenic hypertrophy (SH) was found in up to almost 90% of patients treated with oxaliplatin in various studies, with an average increase in volume of approximately 31% (14-18). Therefore, volumetric measurements of the spleen could correlate with SOS and serve as an indirect measurement of liver damage.
Objective
This study investigates the potential of preoperative SH following chemotherapy, as a predictor of postoperative complications in patients undergoing curative-intent major liver resection for CRLM. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-121/rc).
Methods
Patient cohort and inclusion criteria
This retrospective study included consecutive patients with CRLM undergoing major elective liver resections at the University Hospital RWTH Aachen, between 2010 and 2021. Patients were included if undergoing major resection in curative intent, after preoperative chemotherapy. This was defined as the removal of three or more liver segments, according to the Brisbane classification (19). All patients included in the study initially had unresectable CRLM due to insufficient remaining parenchyma post-hepatectomy. We performed conversion therapy according to the European Society of Medical Oncology (ESMO) guidelines algorithm with the intention to achieve resectability (20). Exclusion criteria included recurrent resections, missing radiological images before or after preoperative chemotherapy, missing data regarding systemic therapy regimens, and prior splenectomy. The primary endpoint of the study was the occurrence of postoperative liver-specific complications (LSC). These included bile leakage/bilioma (25 cases), post-hepatectomy bleeding and coagulopathy requiring red cell concentrate and/or fresh frozen plasma, PPSP, and vitamin K (21 cases), icterus/cholestasis (17 cases), ascites (12 cases), post-hepatectomy liver failure (4 cases), liver metabolism insufficiency with lactate acidosis (3 cases), bile duct fistula (3 cases), hepatorenal syndrome (1 case), subhepatic abscess (1 case), and liver vein thrombosis (1 case). The International Study Group of Liver Surgery definition of PHLF was used for this study, consisting of an increased international normalized ratio (INR) and hyperbilirubinemia on or after postoperative day 5 (21,22). Secondary endpoints were the development of major postoperative complications according to the Clavien-Dindo score (CD ≥3) and severe cumulative morbidity with a Comprehensive Complication Index (CCI) greater than 50 (23).
The study was conducted under the ethical approval of the Institutional Review Board of the RWTH Aachen University (EK-001/21) and in accordance with the current version of the Declaration of Helsinki (as revised in 2013), the Declaration of Istanbul, and good clinical practice guidelines. Informed consent was waived due to the retrospective study design and collection of readily available clinical data.
Data collection
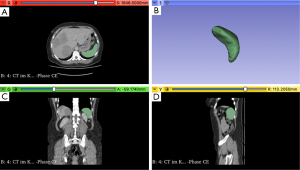
Data were collected from a prospectively-maintained retrospective database (19). This included body mass index (BMI), American Society of Anesthesiologists (ASA) status, systemic therapy protocols, and histopathological characteristics such as R-status (denoted R0 for radical, otherwise R+), tumor/lymph node/metastasis system classification based on Union for International Cancer Control guidelines, and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status. Metastatic lesions detected within three months of primary tumor diagnosis were defined as synchronous. Computerized tomography (CT) and magnetic resonance imaging (MRI) scans from before and after preoperative systemic therapy were identified and exported from the hospital’s Picture Archiving and Communication System. Three-dimensional volumetric measurements of the spleen were conducted using 3D Slicer v5.2.2., an open-source medical imaging visualization application (http://www.slicer.org, Figure 1). By analyzing axial radiographic images, this software enabled precise quantification of spleen volume based on Hounsfield units (HU), using the portal venous contrast medium phase. The software automatically identified and segmented the spleen, while any imprecisions were manually corrected. Spleen volume in cm3 was measured before and after chemotherapy and quantified in terms of relative percentage increase.
Operative technique
Operative technique adhered to previously described protocols (24). Briefly, the Cavitron Ultrasonic Surgical Aspirator (CUSA®, Integra LifeSciences, Plainsboro NJ, USA) was used for parenchymal transection, with clipping or ligation of vascular and biliary structures in open surgery. In minimally invasive resections, either the THUNDERBEAT (Olympus K.K., Tokyo, Japan) or HARMONIC ACE® (Ethicon Inc. Somerville, NJ, USA) systems were used, combined with ECHELON™ vascular staplers (Ethicon, Somerville, New Jersey, USA) or Weck® Hem-o-lok® polymer clips (Teleflex Inc., Pennsylvania, USA). Intermittent Pringle maneuvers were carried out as needed. Radicality of tumor resection was controlled through frozen section. Anesthesiologic management aimed for a low central venous pressure (CVP) during the resection phase.
Statistical analysis
The Kolmogorov-Smirnov test was used to test data distribution normality. Continuous variables are reported as medians with interquartile range (IQR, given as 1st – 3rd quartiles), whereas categorical and ordinal variables are expressed as absolute and relative frequencies. Comparative analysis between groups was conducted using Mann-Whitney U, Chi-square, Pearson’s correlation coefficient or Fisher’s exact tests. All P values <0.05 were considered statistically significant. Independent risk factors for postoperative complications were identified through univariable and multivariable logistic regression analyses, with corresponding odds ratios (OR) and 95% confidence intervals (CI) provided. Variables with P<0.10 were considered for inclusion in the multivariable analysis. Receiver-operating characteristic (ROC), area under the curve (AUC), and Youden Index (YI) analyses were performed to determine the optimal SH cut-off for predicting postoperative complications. Sub-group analysis was carried out for overweight patients (BMI >25 kg/m2). Statistical analysis was performed using SPSS Statistics v29 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 245 patients with CRLM underwent major hepatectomy during the study period, of which 69 were excluded due to inadequate radiographic images, incomplete systemic therapy data, or prior splenectomy. Of the remaining 176 patients, 115 patients received preoperative chemotherapy and were included in the study (Figure 2). Among these, 78 (68%) received oxaliplatin-based chemotherapy. The median duration of preoperative chemotherapy was 6 cycles. In addition, anti-vascular endothelial growth factor (VEGF) immunotherapy and anti-epidermal growth factor receptor (EGFR) therapy was administered in 43 (37%) and 37 (32%) patients, respectively. Preoperative portal vein embolization (PVE) was performed in 50 (44%) cases. Staged resection was executed in 48 (42%) cases, while 20 (17%) patients underwent Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Details of the patient cohort are outlined in Table 1.
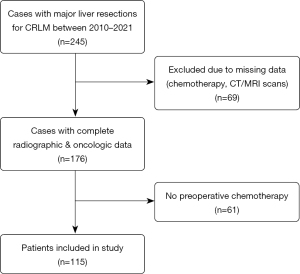
Table 1
| Variable | All patients (n=115) | SH >8.6% (n=62) | SH ≤8.6% (n=53) | P value |
|---|---|---|---|---|
| Age, years | 60 [52–66] | 60 [51–66] | 59 [52–67] | 0.76 |
| Sex (male) | 70 (61%) | 37 (60%) | 33 (62%) | 0.78 |
| BMI, kg/m2 | 25.0 [22.0–28.0] | 24.0 [22.75–27.0] | 26.0 [22.0–28.0] | 0.27 |
| ASA score | 0.89 | |||
| I | 5 (4%) | 3 (5%) | 2 (4%) | |
| II | 46 (40%) | 26 (42%) | 20 (38%) | |
| III | 59 (51%) | 31 (50%) | 28 (53%) | |
| IV | 5 (4%) | 2 (3%) | 3 (6%) | |
| Primary tumor location | 0.10 | |||
| Coecum | 3 (3%) | 3 (5%) | 0 (0%) | |
| Ascending colon | 21 (18%) | 16 (26%) | 5 (9%) | |
| Transverse colon | 3 (3%) | 1 (2%) | 2 (4%) | |
| Descending colon | 6 (5%) | 2 (3%) | 4 (8%) | |
| Sigmoid colon | 33 (29%) | 16 (26%) | 17 (32%) | |
| Rectum | 49 (43%) | 24 (39%) | 25 (47%) | |
| Synchronous metastases | 97 (84%) | 53 (86%) | 44 (83%) | 0.72 |
| More than 3 metastases | 45/95 (47%) | 29/54 (54%) | 16/41 (39%) | 0.16 |
| KRAS status (mutated) | 38/95 (40%) | 24/46 (52%) | 14/49 (29%) | 0.02 |
| Portal vein embolization | 50 (44%) | 26 (42%) | 24 (45%) | 0.72 |
| Preop. platelet counts (/nL) | 232 [173–293] | 199 [145–271.5] | 262 [207.5–334] | <0.001 |
| Preop. albumin (g/dL) | 4.2 [3.6–4.5] | 4.15 [3–4.5] | 4.2 [3.8–4.5] | 0.29 |
| Preop. chemotherapy cycles | 6 [5–8] | 6 [5–10] | 6 [6–8] | 0.75 |
| Oxaliplatin | 78 (68%) | 52 (84%) | 26 (49%) | <0.001 |
| Anti-VEGF | 43 (37%) | 27 (44%) | 16 (30%) | 0.09 |
| Anti-EGFR | 37 (32%) | 13 (21%) | 24 (45%) | 0.009 |
| Staged resection | 48 (42%) | 28 (45%) | 20 (38%) | 0.42 |
| ALPPS | 20 (17%) | 15 (24%) | 5 (9%) | 0.04 |
| Liver R0 | 100 (87%) | 53 (86%) | 47 (89%) | 0.61 |
| Previous liver resection/ablation | 17 (15%) | 11 (18%) | 6 (11%) | 0.33 |
| Liver fibrosis | 58 (50%) | 36/50 (72%) | 22/44 (52%) | 0.03 |
| Operating time (min) | 250.5 [210–333] | 246.5 [192–299] | 270.5 [216–350] | 0.12 |
Values are given as median [1st quartile–3rd quartile] or absolute and relative frequencies. BMI, body mass index; ASA, American Society of Anesthesiology; KRAS, Kirsten rat sarcoma viral oncogene homolog; Anti-VEGF, anti-vascular endothelial growth factor; Anti-EGFR, anti-epidermal growth factor; SH, splenic hypertrophy; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
Splenic hypertrophy was shown to be predictive of LSC in the ROC analysis (AUC =0.62, P=0.02) and a threshold of SH =8.6% (YI =0.25) was defined for the identification of patients at risk (Figure 3). No significant predictive capability was shown for SH regarding complications CD ≥3 (AUC =0.583, P=0.13) or CCI >50 (AUC =0.619, P=0.07). There were no significant differences in age, gender, BMI, ASA status or primary tumor location between patients with SH >8.6% (n=62, 54%) and the rest. Patients with SH>8.6% were significantly more likely to have received oxaliplatin (P<0.001) and exhibited higher rates of LSC (P=0.007), liver fibrosis (P=0.03), and preoperative thrombocytopenia (P<0.001), while undergoing ALPPS significantly more often (P=0.04). Additionally, KRAS mutations were more frequent in this group (P=0.02) and the rate of anti-EGFR systemic therapy was concordantly lower (P=0.009). Differences between groups defined through the SH cutoff are summarized in Table 1. Perioperative outcomes are summarized in Table 2. Here, the only significant difference between the two groups is seen in LSC, where higher rates were observed in the SH >8.6% group (63% vs. 38%, P=0.007).
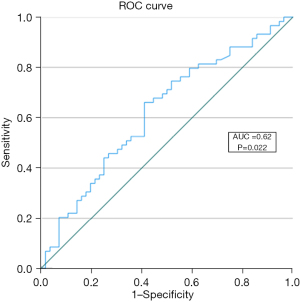
Table 2
| Outcome | All patients (n=115) | SH >8.6% (n=62) | SH ≤8.6% (n=53) | P value |
|---|---|---|---|---|
| Intraop. transfusions (units) | 1 [0–6] | 1 [0–4.25] | 2 [0–6] | 0.58 |
| Complications (Clavien-Dindo) | ||||
| CD1 | 87 (76%) | 44 (71%) | 43 (81%) | 0.21 |
| CD2 | 60 (52%) | 36 (58%) | 24 (45%) | 0.17 |
| CD3a | 38 (33%) | 21 (34%) | 17 (32%) | 0.84 |
| CD3b | 20 (17%) | 13 (21%) | 7 (13%) | 0.27 |
| CD4a | 14 (12%) | 6 (10%) | 8 (15%) | 0.38 |
| CD4b | 6 (5%) | 5 (8%) | 1 (2%) | 0.14 |
| CD≥3 | 52 (45%) | 30 (48%) | 22 (42%) | 0.46 |
| Liver-specific complications | 59 (51%) | 39 (63%) | 20 (38%) | 0.007 |
| Median CCI | 27.6 [17.3–47.3] | 26.2 [20–50.9] | 27.6 [12.2–44.2] | 0.70 |
| CCI >50 | 26 (23%) | 16 (26%) | 10 (19%) | 0.38 |
| POD5 serum bilirubin (mg/dL) | 0.86 [0.505–1.52] | 0.875 [0.54–1.53] | 0.850 [0.43–1.54] | 0.52 |
| POD5 INR | 1.09 [1.04–1.2575] | 1.08 [1.04–1.23] | 1.11 [1.04–1.27] | 0.99 |
| Length of ICU stay (days) | 1 [1–2] | 1.0 [1–2] | 1 [1–2] | 0.94 |
| Length of hospital stay (days) | 11 [8–25] | 12.5 [8.75–25] | 10 [8–24] | 0.25 |
Values are given as median [1st quartile–3rd quartile] or absolute and relative frequencies. SH, splenic hypertrophy; CD, Clavien Dindo; CCI, Comprehensive Complication Index; POD5, postoperative day 5; INR, international normalized ratio; ICU, intensive care unit.
Logistic regression analysis identified SH >8.6% (OR =2.798, 95% CI: 1.312–5.968, P=0.008), the administration of oxaliplatin (OR =2.240, 95% CI: 1.005–4.996, P=0.049), staged resection (OR =2.184 95% CI: 1.023–4.662, P=0.043), and previous liver resection or ablation (OR =3.674, 95% CI: 1.119–12.062, P=0.03) as significant risk factors for LSC. Multivariable analysis confirmed SH>8.6% (OR =2.859, 95% CI 1.104–7.402, P=0.03) as an independent risk factor for LSC (Table 3).
Table 3
| Perioperative factor | Univariable log regression analysis | Multivariable log regression analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Metachronous metastases | 0.722 (0.262–1.984) | 0.53 | |||
| Age | 1.013 (0.978–1.048) | 0.48 | |||
| Sex (female) | 0.853 (0.403–1.805) | 0.68 | |||
| BMI | 1.010 (0.932–1.095) | 0.80 | |||
| BMI >25 kg/m2 | 0.897 (0.431–1.865) | 0.77 | |||
| ASA score | 0.859 (0.488–1.512) | 0.60 | |||
| Cycles of chemotherapy | 1.024 (0.939–1.116) | 0.60 | |||
| Spleen growth (%) | 3.705 (0.937–14.642) | 0.06 | |||
| Oxaliplatin received | 2.240 (1.005–4.996) | 0.049 | |||
| SH >8.6% | 2.798 (1.312–5.968) | 0.008 | 2.859 (1.104–7.402) | 0.03 | |
| Immunotherapy | 0.759 (0.334–1.724) | 0.51 | |||
| Portal vein embolization | 1.611 (0.766–3.390) | 0.21 | |||
| KRAS (mutated) | 0.782 (0.343–1.781) | 0.56 | |||
| Preop. platelets <150/nL | 1.532 (0.574–4.085) | 0.39 | |||
| Liver R0 | 0.667 (0.221–2.013) | 0.47 | |||
| Previous liver resection/ablation | 3.674 (1.119–12.062) | 0.03 | 3.648 (0.899–14.806) | 0.07 | |
| Preop. albumin (g/dL) | 0.319 (0.153–0.665) | 0.002 | 0.458 (0.198–1.062) | 0.07 | |
| Primary tumor location | 0.996 (0.788–1.259) | 0.97 | |||
| Staged resection | 2.184 (1.023–4.662) | 0.043 | 2.057 (0.709–5.966) | 0.18 | |
| Fibrosis | 1.607 (0.694–3.722) | 0.27 | |||
| Operating time | 0.997 (0.992–1.001) | 0.19 | |||
| Surgical technique (lap. vs. open) | 1.000 (0.997–1.003) | 0.97 | |||
| Intraop. transfusions (units) | 1.054 (0.972–1.144) | 0.20 | |||
| Simultaneous resection liver & primary tumor | 0.946 (0.183–4.898) | 0.95 | |||
Factors with P<0.1 in the univariable analysis were considered for inclusion in the multivariable regression model. To avoid a multicollinearity effect, not all eligible variables were included in the multivariable logistic regression analysis. OR, odds ratio; CI, confidence interval; BMI, body mass index; ASA, American Society of Anesthesiology; SH, splenic hypertrophy; KRAS, Kirsten rat sarcoma viral oncogene homolog.
Overweight patients (BMI >25 kg/m2)
An additional subgroup analysis was carried out in overweight patients (n=60, 52%). These patients were more frequently male (72% vs. 49%, P=0.01) and received intraoperative transfusions more often (P=0.03) (Tables S1,S2).
The ROC analysis demonstrated a good predictive capability of SH for all three endpoints: LSC (AUC =0.74, P=0.001), CD ≥3 (AUC =0.67; P=0.03), and CCI >50 (AUC =0.74; P=0.006) (Figures S1-S3). The corresponding cutoff values were 8.38% for LSC (YI =0.467), 20.06% for CD ≥3 (YI =0.37), and 25.15% for CCI >50 (YI =0.53).
Comprehensive details regarding differences between patient groups, as defined by the three cut-off values, can be found in Tables S3-S5. Patients with SH exceeding these cutoffs exhibited higher rates of postoperative LSC and general complications, as wells as higher CCI scores (Tables S6-S8). Logistic regression analysis was performed to assess risk factors for LSC (Tables S9). Our determined cut-off value for LSC was found to be an independent predictor of the corresponding endpoint, namely SH >8.38% for LSC (OR 7.355, 95% CI: 2.257–23.967, P<0.001).
Discussion
Key findings
In this study, we investigated the predictive ability of preoperative chemotherapy-induced SH, with regard to complications after major liver surgery for CRLM. We demonstrated a good predictive ability for LSC in the main cohort, as well as morbidity in terms of CD ≥3 and CCI >50 in overweight patients. Furthermore, a significant association was found between SH and oxaliplatin administration, reduced preoperative platelet counts, and increased liver fibrosis.
Strengths and limitations
Certain study limitations should be taken into account when interpreting our results. The retrospective design excluded patients with unsuitable radiographic images or missing information on chemotherapy regimens. Histological examination results for hepatectomy specimens (e.g., steatosis, fibrosis, SOS) were incomplete. In very few instances, spleen volume measurements had to be conducted using MRI scans, as CT scans were not available, which may have affected measurement accuracy. Our cohort size, despite favorable comparisons to other studies in the literature, is still limited. Thus, further explicit sub-group analysis regarding KRAS mutations, ALPPS or staged resections could not be conducted with confidence in the reliability of the results. Furthermore, the intervals between pre- and post-chemotherapy CT scans were not standardized and chemotherapy regimens varied between patients. Additionally, many patients received their chemotherapy and CT scans at external facilities, resulting in inconsistent timing of spleen volume measurements. Another critical consideration is that some patients may have undergone ablations before or after their major hepatectomy, potentially exerting additional influences on liver function and outcomes. However, these discrepancies reflect the complexities encountered in clinical practice, especially when utilizing multimodal strategies to treat CRLM.
Comparison with similar research
Despite the advantages of preoperative chemotherapy in CRLM patients, a well-known side-effect is CALI, which can be categorized into two forms: non-alcoholic fatty liver disease (NAFLD), leading to ‘yellow liver’, and SOS, referred to as ‘blue liver’ based on its macroscopic features (2,9,25). Previous research has associated NAFLD with the use of 5-fluorouracil (5-FU) and Irinotecan (2,4), whereas oxaliplatin has been shown to damage sinusoidal endothelial cells, resulting in hepatic congestion, sinusoidal dilatation and eventually SOS (9,12,26). This leads to portal hypertension, destruction of hepatic tissue and nodular regeneration, and may contribute to an escalation in perioperative complications (7,27,28). Since SH is a consequence of portal hypertension, it serves as an indicator of liver injury and may provide an explanation for the significant increase in postoperative LSC observed in our study among patients who exceeded our determined cut-off. Patients with CALI may have a reduced future liver remnant function, despite the same absolute volume compared to healthy liver parenchyma, increasing the risk of LSC, particularly PHLF (29).
To our knowledge, our study is the only one to examine post-chemotherapy SH as a predictor of morbidity after major liver resection for CRLM, in the modern era, and with a sizeable cohort. A previous study by Simpson et al. reported similar results, but their chemotherapy cohort was limited to 40 patients, minor resections were included, and postoperative morbidity was not analyzed based on CD or CCI (27). Furthermore, as they included patients up to 2007, it is important to note, that some advances in surgery (e.g., ALPPS, which is prominent in our cohort) and chemotherapy occurred after the end of their study (27). Saez-Carlin et al. reported on 65 patients, who underwent a mixture of atypical, minor, and major resections and of which only 41 received preoperative chemotherapy. They reported no association between SH and complications, but they did not define a cut-off for SH, and do not provide enough data on the statistical analysis (30). Finally, Konishi et al. reported increased rates of complications and liver failure in patients with SH ≥20%, but only included 43 patients with preoperative chemotherapy, of which only 26 underwent major resections (13).
Furthermore, our observations align with studies showing an association between chemotherapy-induced SH and thrombocytopenia, which could serve as another predictor of severe SOS (16,31,32). Moreover, our study revealed a higher incidence of fibrosis in hepatectomy specimens within the SH >8.6% group, reflecting a component of the pathological changes observed in SOS. Interestingly, we observed a significantly higher proportion of patients undergoing ALPPS in the SH >8.6% group, which could be attributed to more advanced disease, necessitating longer and more intensive preoperative chemotherapy, as well as more radical surgery. Considering that the rate of PVE was similar in both groups, the higher rate of ALPPS could also be explained as an attempt to further stimulate FLR growth in cases of failed post-PVE hypertrophy. This could be attributed to CALI. Other than that, we found elevated preoperative albumin values to be protective factor against LSC, as low serum levels are also a sign of impaired liver function. Finally, previous liver surgery was identified as an independent risk factor for LSC, which could be attributed to the already reduced FLR in these patients.
Explanations of findings
Our findings support previous observations that patients treated with oxaliplatin tend to show a significant increase in spleen volume after the last cycle of chemotherapy, compared to those undergoing oxaliplatin-free chemotherapy (14,18,31). As previously reported (9), no significant association between the number of chemotherapy cycles and increase in spleen volume was found in our study.
Our sub-group analysis focused on overweight patients. Our analysis did not find a significant difference in SH rates in overweight patients, compared to the main cohort and the calculated SH cut-off value for the prediction of LSC in overweight patients was similar (≈8%). However, the predictive accuracy for LSC was higher (AUC 0.74 vs. 0.68) in this sub-group, and a strong predictivity for major complications (CD ≥3) and severe cumulative morbidity (CCI >50) was found when SH exceeded 20% in these patients. Previous findings show that a higher BMI is associated with steatosis, CALI and hepatic dysfunction (2,33). Our results suggest, that SH could be used to identify overweight patients with preexisting liver damage, who are at increased risk of post-hepatectomy complications, through cumulative CALI. However, further studies are necessary to precisely examine these effects, with a larger cohort and rigorous analysis of accompanying histological data (e.g., SOS, NAFLD, and fibrosis).
Implications and actions needed
Despite limitations, this study emphasizes the significance of identifying CALI and SOS in patients with CRLM undergoing surgery and the need for comprehensive evaluation by a multidisciplinary team to ensure the best possible care and minimize postoperative morbidity. Patients, particularly those who are obese and receive preoperative chemotherapy, should be closely monitored for SH, as this may warn of increased risk of postoperative complications.
In cases of SH >8.6%, the chemotherapy regimen could be adjusted or paused, while alternative methods to surgery alone could be discussed, such as combined resection with local ablation. These patients might particularly benefit from minimally invasive surgery and/or parenchyma-sparing techniques, instead of major resection. Postoperatively, patients could be monitored longer on the ICU and more closely on the normal ward, allowing for early intervention in cases of LSC.
Conclusions
Preoperative, post-chemotherapy SH can be used to identify patients at risk of postoperative LSC, after major liver resection for CRLM. Patients with SH >8.6% are particularly at risk of LSC. In overweight patients (BMI >25 kg/m2), SH >20% is additionally associated with major complications (CD ≥3). Further studies are necessary to provide valuable insights into the underlying mechanisms of SOS/CALI and to explore preventive strategies to mitigate and treat such injury. Ultimately, these efforts could lead to improved postoperative outcomes in patients with CRLM.
Acknowledgments
Funding: This research project was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-121/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-121/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-121/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-121/coif). D.T. reports honoraria from Bayer AG for lectures. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted under the ethical approval of the Institutional Review Board of the RWTH Aachen University (EK-001/21) and in accordance with the current version of the Declaration of Helsinki (as revised in 2013), the Declaration of Istanbul, and good clinical practice guidelines. Informed consent was waived due to the retrospective study design and collection of readily available clinical data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Osterlund P, Salminen T, Soveri LM, et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg Health Eur 2021;3:100049. [Crossref] [PubMed]
- Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007;94:274-86. [Crossref] [PubMed]
- Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepatobiliary Pancreat Surg 2008;15:570-80. [Crossref] [PubMed]
- Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065-72. [Crossref] [PubMed]
- Lam VW, Spiro C, Laurence JM, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol 2012;19:1292-301. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118-24. [Crossref] [PubMed]
- Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr 2014;3:238-46. [PubMed]
- Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460-6. [Crossref] [PubMed]
- Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1-7. [Crossref] [PubMed]
- Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology 2008;134:1670-81. [Crossref] [PubMed]
- Slade JH, Alattar ML, Fogelman DR, et al. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer 2009;8:225-30. [Crossref] [PubMed]
- Konishi T, Yoshidome H, Shimizu H, et al. Splenic enlargement induced by preoperative chemotherapy is a useful indicator for predicting liver regeneration after resection for colorectal liver metastases. World J Surg Oncol 2020;18:139. [Crossref] [PubMed]
- Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 2010;28:2549-55. [Crossref] [PubMed]
- Jung EJ, Ryu CG, Kim G, et al. Splenomegaly during oxaliplatin-based chemotherapy for colorectal carcinoma. Anticancer Res 2012;32:3357-62. [PubMed]
- Kim MJ, Han SW, Lee DW, et al. Splenomegaly and Its Associations with Genetic Polymorphisms and Treatment Outcome in Colorectal Cancer Patients Treated with Adjuvant FOLFOX. Cancer Res Treat 2016;48:990-7. [Crossref] [PubMed]
- Park S, Kim HY, Kim H, et al. Changes in Noninvasive Liver Fibrosis Indices and Spleen Size During Chemotherapy: Potential Markers for Oxaliplatin-Induced Sinusoidal Obstruction Syndrome. Medicine (Baltimore) 2016;95:e2454. [Crossref] [PubMed]
- El Chediak A, Haydar AA, Hakim A, et al. Increase in spleen volume as a predictor of oxaliplatin toxicity. Ther Clin Risk Manag 2018;14:653-7. [Crossref] [PubMed]
- Amygdalos I, Müller-Franzes G, Bednarsch J, et al. Novel machine learning algorithm can identify patients at risk of poor overall survival following curative resection for colorectal liver metastases. J Hepatobiliary Pancreat Sci 2023;30:602-14. [Crossref] [PubMed]
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10-32. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Amygdalos I, Hitpass L, Schmidt F, et al. Survival after combined resection and ablation is not inferior to that after resection alone, in patients with four or more colorectal liver metastases. Langenbecks Arch Surg 2023;408:343. [Crossref] [PubMed]
- White MA, Fong Y, Singh G. Chemotherapy-Associated Hepatotoxicities. Surg Clin North Am 2016;96:207-17. [Crossref] [PubMed]
- Chun YS, Laurent A, Maru D, et al. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol 2009;10:278-86. [Crossref] [PubMed]
- Simpson AL, Leal JN, Pugalenthi A, et al. Chemotherapy-induced splenic volume increase is independently associated with major complications after hepatic resection for metastatic colorectal cancer. J Am Coll Surg 2015;220:271-80. [Crossref] [PubMed]
- Zhao J, van Mierlo KMC, Gómez-Ramírez J, et al. Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br J Surg 2017;104:990-1002. [Crossref] [PubMed]
- Chapelle T, Op De Beeck B, Huyghe I, et al. Future remnant liver function estimated by combining liver volumetry on magnetic resonance imaging with total liver function on (99m)Tc-mebrofenin hepatobiliary scintigraphy: can this tool predict post-hepatectomy liver failure? HPB (Oxford) 2016;18:494-503. [Crossref] [PubMed]
- Saez-Carlin P, García-Botella A, Diez-Valladares LI, et al. Splenic volume as a biomarker of hepatic damage after chemotherapy in patients with resected colorectal liver metastases (CRLM). Clin Transl Oncol 2020;22:1180-6. [Crossref] [PubMed]
- Miyata T, Takamura H, Kin R, et al. Spleen Volume as a Predictive Biomarker for Thrombocytopenia and Liver Dysfunction After Oxaliplatin-based Chemotherapy. Anticancer Res 2020;40:3361-70. [Crossref] [PubMed]
- Soubrane O, Brouquet A, Zalinski S, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg 2010;251:454-60. [Crossref] [PubMed]
- Lv Y, Ding XS, Li Y, et al. High BMI and low HDL-C predict the chemotherapy-related hepatic dysfunction in Chinese advanced NSCLC patients. Cancer Biomark 2016;16:89-97. [Crossref] [PubMed]


