
Evaluating liver resection outcomes post Y90 TARE with personalized dosimetry in intermediate or advanced hepatocellular carcinoma: a focus on surgical and biliary complications
Highlight box
Key findings
• A shorter time interval (less than 6 months) between transarterial radioembolization (TARE) and surgery poses a higher risk of bile leaks in poor prognosis hepatocellular carcinoma (HCC) patients compared to liver resections performed after waiting at least 6 months post-TARE.
What is known and what is new?
• Post hepatectomy liver failure is a known post operative complication following liver resection. TARE is known to adversely affect the biliary system.
• This manuscript is the first to report the time interval between TARE and surgery as a risk factor for bile leaks in a homogenous population of HCC patients.
What is the implication, and what should change now?
• Our findings indicate that waiting at least 6 months before performing liver resection following TARE strongly reduces the risk of postoperative bile leaks in addition to other known benefits: better patient selection with less aggressive tumor biology, optimal future liver remnant (FLR) increase, and maximization of radiation effects on the tumor.
Introduction
Transarterial radioembolization (TARE) has increasingly been recognized as a key treatment modality for hepatocellular carcinoma (HCC), transitioning from a palliative approach in advanced stages to a versatile therapy across various HCC stages, as recognized in the 2022 Barcelona Clinic Liver Cancer (BCLC) guidelines (1). Several institutions now consider it as a first line transarterial locoregional therapy for HCC patients (2).
The role of surgery in treating HCC has evolved. Liver resection is well-established for early-stage HCC, but its role in treating intermediate or advanced HCC remains to be clearly defined. Using TARE as bridge or downstaging therapy in poor-prognosis intermediate or advanced HCCs has shown promising outcomes in observational studies (3-5). In this context, the risk of post hepatectomy liver failure (PHLF), often linked to inadequate future liver remnant (FLR) represents the main cause of death (6,7), explaining why it has been investigated first. By inducing atrophy in the tumor-bearing lobe and hypertrophy in the remaining liver, radiation lobectomy potentially expands surgical candidacy for those previously deemed unsuitable due to insufficient FLR (8,9).
While PHLF as a peri operative complication post-TARE is well documented, there is a paucity of data on other peri operative complications in HCC patients undergoing liver resection after TARE. Bile leaks, for instance, have been frequently observed in studies focusing on PHLF outcomes in TARE-treated HCC patients undergoing liver resection (9-11). Moreover, TARE is known to adversely affect the biliary system leading to complications such as biliary necrosis, biloma, cholecystitis, abscess and stricture (12).
Therefore, considering the literature gap regarding PHLF and other peri operative complications and hypothesizing radiation-induced weakening of the biliary system, the present study was aimed to document the incidence and severity of biliary complications in HCC patients undergoing liver resection after TARE, and to identify potential risk factors that could be mitigated before surgery. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-151/rc).
Methods
Patient selection
This study adhered to the principles of the Declaration of Helsinki (as revised in 2013) and was approved by our institutional review board (IRB CHU de Montpellier 202301480) and written informed consent was obtained from all patients.
We enrolled consecutive patients who underwent TARE with Y90 glass microspheres between June 2015 and December 2022. TARE was indicated for non-resectable early stage BCLC A patients, BCLC B or C patients with portal vein tumor thrombosis (PVTT). Exclusion criteria for TARE were non-preserved liver function (Child-Pugh ≥B7), unilobar disease with post treatment inadequate hepatic reserve (<30%), Eastern Cooperative Oncology Group (ECOG) score >1, elevated bilirubin level (>2 mg/dL), severe renal impairment [glomerular filtration rate (GFR) <30 mL/min/1.73 m2], presence of extrahepatic spread, and bilobar disease without curative options for the contralateral disease. Inclusion criteria were: all HCC, diagnosed histopathologically or by European Association for the Study of the Liver (EASL) imaging criteria, undergoing TARE with Y90-loaded glass microspheres followed by surgery. Patients undergoing liver transplantation were excluded to focus on surgical complications following liver resection.
Radioembolization
All patients underwent angiography and 99m-Technetium macroaggregated albumin (MAA) SPECT before receiving Y90-loaded glass microspheres for TARE, ensuring accurate tumor (and PVTT, if present) targeting while minimizing damage to healthy liver tissue. The lung shunt fraction was calculated, and the distribution of MAA outside the liver was confirmed. The Medical Internal Radiation Dosimetry (MIRD) method calculated the necessary Y90 activity to deliver at least 205 Gy to the tumor, preferably >250–300 Gy, while limiting the whole liver dose to <150 Gy, according to the personalized dosimetry concept (13).
Baseline characteristics and follow-up
Baseline imaging, including computed tomography (CT) or magnetic resonance imaging (MRI), was performed within a month before TARE. Follow-up assessments every three months included clinical, biological, and imaging evaluations. Two independent readers assessed imaging responses using the modified Response Evaluation Criteria in Solid Tumors (mRECIST), with discrepancies resolved through consensus. We categorized responses as localized within the treated zone while also considering both localized and distant tumor progression.
Surgery
Our local policy was to consider surgery after TARE when: no tumor progression was observed after a minimum 3-month follow-up period, and adequate future liver remnant (standardized FLR ≥40% in cirrhosis, ≥30% otherwise) was achieved. In cases where a right hepatectomy was indicated, the FLR volume was calculated during each follow-up visit after Y90 radioembolization treatment to assess hypertrophy. Surgery was considered if adequate hypertrophy was observed. Each case was discussed during our bi-weekly multidisciplinary tumor board, comprising interventional radiologists, hepatologists, oncologists, and liver surgeons. In case of disease progression in the untargeted TARE region, patients were evaluated for additional (ideally intraoperative) thermal ablation. If the latter was not feasible, consideration was given to transarterial chemoembolization (TACE) as an option to potentially downstage patients for curative treatment. Experienced liver surgeons performed the resections, categorized as minor (one or two Couinaud’s segments), major (three to four segments), or major extended (five or more segments). Parenchymal dissections were performed using CUSA Dissectron® (Integra Life Sciences Corp., Tokyo, Japan) in all cases of resection.
Surgical data
The following were collected: the time interval from TARE to surgery, operative time, blood loss and surgical difficulties (as recorded in the surgical report). Pre operative biological parameters such as white blood cell counts, bilirubin levels, international normalized ratios (INR), albumin levels, gamma-glutamyltransferase (GGT) and alpha-fetoprotein (AFP) were also noted. Postoperative data encompassed hospital stay, morbidity [Dindo-Clavien classification (14)], PHLF based on the International Study Group of Liver Surgery (ISGLS) criteria (6), bilirubin levels, INR on day 5 after surgery, 90-day mortality, infectious complications, blood transfusion and CT scans during the hospital stay. We paid special attention to biliary complications and classified bile leaks according to the ISGLS criteria (15). We also investigated long-term postoperative complications, defined as those occurring during the first two quarterly visits following surgery, i.e., at 3 months and 6 months. Data were retrieved from consultation reports during follow-up visits with surgeons. This included any symptom likely to impact quality of life, such as chronic pain, as well as complications directly associated with the surgical procedure itself, such as post-operative eventration.
Statistical analysis
Continuous variables were presented as median with interquartile range (IQR) values and categorical values as percentages. Variable selection for the logistic regression model was informed by literature data. Univariate analysis was conducted for each selected explanatory variable. Multivariate analysis was then performed using a stepwise method. A robust variance estimator was used systematically. Model discrimination was assessed via receiver operating characteristic (ROC) curve, with calibration conducted using the bootstrap method. Median follow-up was calculated using the reverse Kaplan-Meier method. Progression-free survival (PFS) and overall survival (OS) were determined using Kaplan-Meier. A P value <0.05 was considered statistically significant. All statistical calculations were executed using R-studio software 2021.09.0 Build 351.
Results
Population (Figure 1)
From June 2015 to December 2022, 154 patients with liver tumors underwent Y90 glass microspheres TARE at our institution. Of these, 123 were treated for HCC. Thirty-five patients (30%) underwent surgical treatment, with 86% (30/35) receiving liver resection and 11% (4/35) undergoing liver transplantation (see Figure 1). Due to a challenging dissection of liver parenchyma, one patient’s surgical plan was modified to intraoperative microwave ablation. Therefore, this study focused on 30 HCC patients who underwent liver resection post-TARE, whose baseline characteristics are detailed in Table 1.
Table 1
| Baseline characteristics | Values |
|---|---|
| Sex | |
| Male | 23 [74] |
| Female | 7 [26] |
| Age (years) | 67 [61–70] |
| Underlying liver disease | |
| OH | 15 [50] |
| HCV | 6 [20] |
| NASH | 4 [13] |
| Other (healthy liver, Alagille syndrome) | 5 [17] |
| Cirrhosis on pre-TARE imaging | |
| Present | 18 [60] |
| Absent | 12 [40] |
| Previous treatments before RE | |
| TACE | 2 [7] |
| Liver resection | 1 [3] |
| Systemic therapy (sorafenib or immunotherapy) | 2 [7] |
| Liver resection + TACE/TACI + systemic therapy | 2 [7] |
| AFP (ng/mL) | 16.2 [7.2–92.6] |
| Index lesion size (mm) | 68 [43–95] |
| Vascular invasion (PVT and/or HVT) | |
| Absent | 12 [40] |
| PV4 | 2 [7] |
| PV3 | 2 [7] |
| PV2 | 6 [20] |
| PV1 | 6 [20] |
| HVT | 3 [10] |
| BCLC | |
| A | 8 [27] |
| B | 4 [13] |
| C* | 18 [60] |
| Treatments after TARE | |
| TACE (in non-treated liver) | 2 [7] |
| TA (in non-treated liver) | 1 [3] |
| Systemic therapy | 2 [7] |
| Portal vein embolization | 2 [7] |
| Pre-operative biological data | |
| Lymphocytic count | 0.9 [0.6–1.2] |
| Neutrophil count | 3.7 [2.7–5.1] |
| Albumin (g/L) | 42 [40–45] |
| GGT (UI/mL) | 129 [81–246] |
| INR | 1.03 [0.99–1.08] |
| Bilirubin levels (mg/L) | 10 [5–54] |
Values are presented as median [interquartile range] or number [percentage]. *, 15 patients with portal vein thrombosis, 2 patients with hepatic vein thrombosis and 1 patient with hepatic vein and portal vein thrombosis. TARE, transarterial radioembolization; RE, radioembolization; HCC, hepatocellular carcinoma; OH, alcoholic liver disease; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; TACE, transarterial chemoembolization; TACI, transarterial chemoinfusion; AFP, alpha-fetoprotein; PVT, portal vein thrombosis; HVT, hepatic vein thrombosis; BCLC, Barcelona Clinic Liver Cancer classification; TA, thermal ablation; GGT, Gamma-glutamyl transferase; INR, international normalized ratio.
Follow-up: radiological response and additional treatment
The objective response rate (ORR) at 3 months post-TARE, based on localized mRECIST criteria, was 97%: comprising 23% complete response (CR), 73% partial response (PR), and 3% stable disease (SD). Prior to surgery, the ORR remained at 97%, with 33% CR, 64% PR, and 3% SD.
Three patients (10%) experienced progressive disease in untreated areas following TARE. One patient underwent intraoperative thermal ablation along with a left hepatectomy. The remaining two received pre-surgical TACE, with one undergoing right hepatectomy and concurrent wedge resection in segment III, while the other underwent monosegmentectomy in segment VI combined with thermal ablation in segment II.
Surgical data (Table 2)
Table 2
| Surgical data | Values |
|---|---|
| Type of surgery | |
| Minor (one or two Couinaud’s segments) | 8 [27] |
| Major (three to four Couinaud’s segments) | 20 [66] |
| Major extended (five or more Couinaud’s segments) | 2 [7] |
| Operative time (minutes) | 388 [314–442] |
| Blood loss (mL) | 675 [325–775] |
| Intraoperative incidents | 4 [13] |
| Intestinal wound | 1 [25] |
| Diaphragm wound | 1 [25] |
| Hemodynamic instability requiring vasoactive drugs | 2 [50] |
| ICU stay (days) | 4 [2–7] |
| Hospital stay (days) | 11 [7–14] |
| Clavien-Dino classification | |
| No complication | 13 [43] |
| Grade 1 | |
| Ascites | 3 [10] |
| Urine retention | 1 [3] |
| Grade 2 | |
| Anemia requiring blood transfusion | 1 [3] |
| Grade 3a | |
| Intra-abdominal collection | 3 [10] |
| Pneumothorax | 1 [3] |
| Pleural effusion | 1 [3] |
| Grade 3b | |
| Bile leak | 2 [7] |
| Evisceration | 1 [3] |
| Wall abscess | 1 [3] |
| Grade 4 | |
| Renal and pulmonary dysfunction | 1 [3] |
| Grade 5 | |
| Death (severe PHLF) | 1 [3] |
| Death (severe peritonitis from an intestinal wound) | 1 [3] |
| PHLF | |
| Absent | 18 [60] |
| A | 1 [3] |
| B | 10 [34] |
| C | 1 [3] |
| Increased bilirubin levels on day 5 or later after surgery | 15 [50] |
| Increased INR on day 5 or later after surgery | 10 [33] |
| 90-day mortality | 2 [7] |
| Antibiotics during hospital stay | 13 [43] |
| Intra-abdominal sepsis | 5 [38] |
| Pulmonary infection | 2 [15] |
| Urinary tract infection | 1 [8] |
| Wall abscess | 1 [8] |
| No infectious call points | 3 [23] |
| Antibiotic prophylaxis | 1 [8] |
| Blood transfusion during post operative stay | 4 [13] |
| CT scan during hospital stay | 21 [70] |
Values are presented as median [interquartile range] or number [percentage]. TARE, transarterial radioembolization; HCC, hepatocellular carcinoma; ICU, intensive care unit; PHLF, post hepatectomy liver failure; INR, international normalized ratio; CT, computed tomography.
Pre and perioperative data
The median interval between TARE and surgery was 6.9 months (IQR: 4.3–10.8 months). Right-sided hepatectomy was performed in 63% (19/30) of the cases. The median operative duration was 388 minutes (IQR: 314–442 minutes), and median blood loss was 675 mL (IQR: 325–775 mL). Forty percent of patients experienced severe postoperative complications (grade 3 or above), including a 90-day mortality rate of 7% (2/30), primarily due to multiorgan failure. One patient developed severe peritonitis following an intestinal injury and another suffered Grade C PHLF.
PHLF
Grade A PHLF was noted in one patient without any clinical implications. Grade B PHLF was observed in 33% of patients, all of whom developed ascites, successfully managed with medical treatment alone. Notably, 90% of patients with grade B PHLF underwent right-sided hepatectomy. Grade C PHLF, leading to death, was observed in one patient.
Dosimetric considerations
Table 3 presents Y90-treatment parameters comparing patients with and without biliary complications. No statistically significant differences were observed regarding tumor location (central vs. peripheral), treatment location (lobar, multisegmental, or segmental), tumor absorbed dose, perfused liver dose, perfused liver volume, perfused normal liver dose and perfused normal liver volume.
Table 3
| Parameters | Bile-leak group (n=6) | Non-bile leak group (n=24) | OR (95% CI) | P |
|---|---|---|---|---|
| Tumor location | 0.591 (0.090, 3.864) | 0.58 | ||
| Central | 4 [67] | 13 [54] | ||
| Peripheral | 2 [33] | 11 [46] | ||
| Tumor absorbed dose (Gy) | 313 [245–320] | 318 [223–395] | 1.003 (0.997, 1.009) | 0.33 |
| Tumor size (mm) | 57 [47–85] | 71 [42–95] | 1.000 (0.975, 1.026) | 0.99 |
| Treatment | – | 0.83* | ||
| Lobar | 3 [50] | 13 [54] | ||
| Multisegmental | 3 [50] | 8 [33] | ||
| Segmental | 0 | 3 [13] | ||
| Perfused liver dose (Gy) | 247 [218–250] | 188 [151–296] | 1.002 (0.995, 1.009) | 0.56 |
| Perfused liver volume (mL) | 539 [284–864] | 683 [437–1,053] | 1.000 (0.998, 1.001) | 0.76 |
| Perfused normal dose (Gy) | 217 [166–220] | 152 [94–195] | 1.009 (0.998, 1.021) | 0.11 |
| Perfused normal volume (mL) | 347 [233–487] | 635 [383–818] | 0.996 (0.992, 1.001) | 0.10 |
Values are presented as median [interquartile range] or number [percentage]. *, the Fisher exact test was used due to the data distribution, rendering logistic regression infeasible. OR, odds ratio; CI, confidence interval.
Furthermore, when considering the potential association between tumor size or location with tumor absorbed dose, no statistically significant differences were observed. Specifically, the interaction between tumor size and tumor absorbed dose did not show a significant association with biliary complications (P=0.13). Similarly, the interaction between tumor location and tumor absorbed dose also did not yield a statistically significant difference (P=0.30).
Biliary complications
Twenty percent of patients (6/30) experienced grade B bile leaks, all after right-sided hepatectomies. These leaks manifested as early as day 2 following surgery in 4/6 cases, and were associated with at least one of the following findings: biliocutaneous fistulas, bilomas and/or intra-abdominal sepsis. Conservative management was successful, with percutaneous and/or endoscopic interventions. Recurrent bile leakage occurred in two patients, with one showing signs of intrahepatic bile duct stenosis on a postoperative day 5 CT scan. One patient died from a biliary complication after a septic shock due to infected biloma. Notably, intraoperative necrosis of the left bile duct was observed in one patient, successfully managed with externalized biliary drainage for 69 days with no biliocutaneous fistulas, biloma or intra-abdominal sepsis following surgery or during later follow-up.
Risk factors for biliary complications (Tables 4,5)
Table 4
| Variable | Bile leak group (n=6) | No bile leak group (n=24) | Univariable analysis, P value |
|---|---|---|---|
| Time to surgery (months) | 4.2 [3.7–5.4] | 8.2 [5.6–11.3] | 0.057 |
| Operative time (min) | 384 [314–442] | 414 [326–437] | 0.81 |
| Blood loss (mL) | 850 [680–1,150] | 500 [300–1,500] | 0.09 |
| Hospital stay (days) | 22 [12–39] | 11 [7–13] | 0.03 |
| ICU stay (days) | 10 [4–19] | 4 [2–6] | 0.03 |
| Major hepatectomy | 6 [100] | 16 [67] | 0.96 |
| Prior treatment | 2 [33] | 5 [21] | 0.52 |
| Age (years) | 73 [69–76] | 66 [59–68] | 0.052 |
| Sex | 0.52 | ||
| Female | 2 [33] | 5 [21] | |
| Male | 4 [67] | 19 [79] | |
| Baseline AFP (ng/mL) | 523 [14–2,561] | 16 [5–70] | 0.68 |
| Baseline lymphocytes count (G/L) | 0.86 [0.64–1.08] | 0.90 [0.60–1.24] | 0.55 |
| Baseline neutrophil count (G/L) | 2.8 [2.7–4.8] | 3.8 [2.8–5.1] | 0.55 |
| Baseline albumin (g/L) | 45 [42–46] | 42 [40–44] | 0.23 |
| Baseline GGT (UI/L) | 113 [83–218] | 129 [84–248] | 0.99 |
| Baseline INR | 1.04 [0.98–1.09] | 1.03 [0.99–1.08] | 0.62 |
| Baseline bilirubin (mg/L) | 8 [7–10] | 10 [9–17] | 0.23 |
| Portal vein invasion | 5 [80] | 11 [46] | 0.13 |
Values are presented as median [interquartile range] or number [percentage]. TARE, transarterial radioembolization; HCC, hepatocellular carcinoma; ICU, intensive care unit; AFP, alpha-fetoprotein; GGT, gamma glutamyl transferase; INR, international normalized ratio.
Table 5
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Time to surgery (3–6 vs. >6 months) | 12.14 (1.19–123.62) | 0.04 | 87.77 (1.06–7,239.07) | 0.047 | |
| Operative time (min) | 1.00 (0.99–1.01) | 0.81 | |||
| Blood loss (mL) | 1.00 (1.00–1.01) | 0.09 | 1.00 (1.00–1.01) | 0.12 | |
| Tumor size (mm) | 1.00 (0.98–1.03) | 0.99 | |||
| Cirrhose (absent vs. present) | 1.68 (0.28–10.09) | 0.58 | |||
| Major hepatectomy (no vs. yes) | <0.001 (<0.001–>999) | 0.96 | |||
| Prior treatment (no vs. yes) | 0.53 (0.074–3.75) | 0.52 | |||
| Age | 1.14 (1.00–1.29) | 0.052 | 1.16 (0.91–1.48) | 0.24 | |
| Sex (female vs. male) | 0.53 (0.074–3.75) | 0.52 | |||
| Baseline AFP (ng/mL) | 1.00 (1.00–1.00) | 0.68 | |||
| Baseline lymphocytes count (G/L) | 0.49 (0.047–5.07) | 0.55 | |||
| Baseline neutrophil count (G/L) | 0.85 (0.49–1.47) | 0.55 | |||
| Baseline albumin (g/L) | 1.18 (0.90–1.56) | 0.23 | |||
| Baseline GGT (UI/L) | 1.00 (0.99–1.01) | 0.99 | |||
| Baseline INR | 0.085 (<0.001–>999) | 0.62 | |||
| Baseline bilirubin | 0.83 (0.62–1.12) | 0.23 | |||
| Portal vein invasion | 0.17 (0.017–1.68) | 0.13 | |||
HCC, hepatocellular carcinoma; TARE, transarterial radioembolization; OR, odds ratio; CI, confidence interval; AFP, alpha-fetoprotein; GGT, gamma glutamyl transferase; INR, international normalized ratio.
In univariate analysis, patients undergoing liver resection between 3 and 6 months after TARE exhibited a 12-fold increase (P=0.04) in the odds of experiencing biliary complications compared to those who underwent surgery after 6 months. Following adjustment for potential confounders, namely age at the time of surgery and blood loss during the procedure, the observed association retained its statistical significance (P=0.047), contrary to patients’ age (P=0.24) and blood loss (P=0.12).
Our statistical model exhibited excellent discriminative performance, boasting an initial apparent area under the curve (AUC) of 0.92, which was subsequently refined to 0.87 following bootstrap calibration.
Infectious complications
Forty percent of patients experienced infectious complications, predominantly intra-abdominal sepsis (19%) and pulmonary infections (7%). In patients with grade B bile leak, 67% developed intra-abdominal infection. Peritonitis occurred in one patient after intestinal injury. Prophylactic and probabilistic antibiotic therapies were administered as needed, based on clinical indicators such as fever and inflammatory syndrome.
Long-term postoperative complications
Long-term postoperative complications, observed during the initial two quarterly follow-up visits, were assessable in all living patients at both 3 and 6 months following surgery. Among them, 18% experienced such complications. Two experienced chronic abdominal pain, with one also reporting abdominal bloating relieved by defecation (Koenig syndrome), potentially due to postoperative adhesions. Other complications included one case of chronic ascites and two cases of postoperative eventration.
Survival outcomes (Figure 2A,2B)
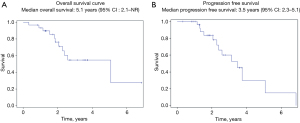
Over a median follow-up period of 2.8 years [95% confidence interval (CI): 1.9–3.6], 13 out of 30 patients (43%) experienced progressive disease following liver resection, while 11 out of 30 patients (37%) died. Median OS in TARE-treated HCC patients undergoing liver resection was 5.1 years (95% CI: 2.1–NR). Median PFS was 3.5 years (95% CI: 2.3–5.1), 42 months (95% CI: 27–60).
Discussion
This study confirms the excellent oncological outcomes following liver resection after Y90 TARE with personalized dosimetry in intermediate/advanced HCC patients (4). Especially, the median OS and PFS are improved compared to those following TARE or surgery alone (16,17). However, postoperative morbidity and surgical difficulty may have been underestimated in this specific population of HCC patients undergoing liver resection after TARE.
One patient could not be resected in our series due to challenging dissection. Perioperative morbidity and mortality were 40% and 7% respectively. Khan et al.’s meta-analysis of patients undergoing liver resection after TARE reported perioperative morbidity and 90-day mortality rate of 26% (95% CI: 16–37%) and 3.0% (95% CI: 0.3–7.4%) respectively (18). Nonetheless, the meta-analysis included studies involving both HCC and non-HCC tumors, with only 7 studies exclusively focused on HCC. Among these, 4 reported BCLC classification, representing 25% with BCLC B or C compared to 73% in our cohort.
When considering biliary complications, these are far from negligible, since they occurred in 20% of our patients. Two-thirds of patients with grade B bile leaks experienced intra-abdominal sepsis and biliary-specific mortality was 9% (1 out of 11 patients), highlighting the critical importance of identifying risk factors of bile leaks. The occurrence of bile leakage in patients subject to liver resection without prior TARE ranges from 4.2% to 19% in recent studies (19-23) when considering both benign and malignant tumors. In surgical series on resection of HCC, the incidence of biliary complications ranges from 5.8–7.2% (24,25). When considering the specific population of TARE-treated patients undergoing liver resection, the incidence rates seems higher, but exhibited a large range: 13–40% (9,26-28). Once again, the populations studied were heterogeneous, encompassing both HCC and non-HCC tumors. Gabr et al. (9) reported a bile leakage incidence of 13% in HCC patients undergoing liver resection after TARE with Y90-loaded glass microspheres. We observed a higher incidence of bile leaks (20%). The median tumor size in our cohort was larger (66 vs. 49 mm), implying larger irradiated volumes. The dosimetric approach was also different. In our study, we conducted a personalized dosimetric approach (contrary to Gabr’s cohort), aiming at delivering a high radiation dose to the tumor whereas in Gabr’s study, a threshold dose was delivered to the segment without evaluating the tumor dose. These differences in terms of baseline tumor spread and dosimetric approach as well as the design of our study focused on biliary complication certainly explain the higher incidence of bile leaks in our study.
Numerous risk factors for postoperative bile leaks have been identified (19-25,29-31): major vs. minor hepatectomy, anatomical vs. non-anatomical resection, repeated hepatectomy, bilio-enteric anastomosis, and operative time being frequently cited. Additional risk factors identified included intraoperative bile leak or drain placement, lymphadenectomy, vascular reconstruction, liver remnant ischemia time of 45 minutes or more, Pringle’s maneuver, liver surface trauma ≥57.5 cm2, portal vein embolization, associating liver partition and portal vein ligation (ALPPS), male gender, body mass index, diabetes mellitus, hemoglobin <10 g/dL, albumin <3.5 g/dL, Child-Pugh grade and macroscopic portal vein invasion. While our final statistical model incorporated blood loss, patient age before surgery and the time interval between TARE and liver resection, only the latter emerged as significantly associated with biliary complications.
There is a great debate on the optimal time point to resect patients after TARE. This result brings new insights and strongly suggest to wait at least 6 months before resecting the patient. In our cohort, patients undergoing surgery after 6 months exhibited a twelve times lower risk of experiencing bile leaks compared to those treated surgically within 3–6 months after TARE. We can make the hypothesis that the waiting period helps to heal radiation-induced bile duct injuries. This minimum 6-month delay not only diminishes the likelihood of biliary complications but has also strong additional benefits: it refines patient selection based on test-of-time excluding patients with aggressive tumor biology (9,32,33), mitigates the risk of PHLF by allowing important FLR growth, and optimizes the cytotoxic effect of radiation (34,35). In addition, this aligns with lower perioperative mortality, as suggested in a recent meta-analysis (18).
Atassi et al. (12) reported an incidence rate of 10% of biliary-related imaging findings after TARE including biliary necrosis, biloma, cholecystitis, gallbladder wall enhancement, gallbladder wall rent, abscess and stricture. The physiopathological hypotheses explaining biliary toxicities after Y90 TARE involve the possibility of a microembolic effect of Y90 particles and/or direct radiation-induced injury (12). In our study population, no disparity in bile leaks was noted between cirrhotic and non-cirrhotic patients, whereas the peribiliary plexus is typically hypertrophied in cirrhotic patients, making them less susceptible to ischemic/chemic insults of bile ducts (36). In addition, the embolic effect of Y90 glass microspheres is very limited (37). Therefore, radiation-induced injury emerges as a plausible primary cause of bile injury in our cohort. The mean and maximum ranges of beta particles in soft tissues are 2.5 and 11 mm respectively (38). Y-90 glass microspheres radioembolization can therefore damage nearby bile ducts if they are within the range of emitted beta particles.
In the bile leak group, tumors were more frequently located centrally, that is, near the hilum. A high perfused normal liver dose theoretically imparts a higher risk to the associated bile ducts. Interestingly, the perfused normal dose was greater in the bile-leak group compared to the non-bile-leak group, which aligns with this theoretical risk However, no statistically significant difference emerged when considering Y90 treatment parameters including tumor absorbed dose and its potential interactions with tumor size and tumor location close to the hilum. This lack of significance may be due to the small sample size (6 patients with bile leaks), which reduces the statistical power.
Strengths of this study include its homogenous population of HCC patients undergoing liver resection and its detailed description of biliary complications in TARE-treated HCC patients undergoing surgery after downstaging of intermediated/advanced disease. To our knowledge, this is the largest cohort of intermediate and advanced HCC patients undergoing liver resection after TARE with personalized dosimetry and the first to identify time from TARE to surgery as a predictive factor associated with developing bile leak. Limitations of our study include the small sample size and its retrospective nature. Another limitation of our study was the inability to examine the relationship between the interval from Y90 calibration to radioembolization and the occurrence of bile leaks. According to Pasciak et al. (39), delaying radioembolization treatment post-calibration could result in heightened normal liver toxicity. This is due to the increased number of microspheres, which promote a more uniform dose distribution and consequently expose a larger volume of the liver—and therefore the bile ducts, especially in centrally located tumors—to radiation. This underscores the need for further studies to better understand this relationship and its implications for patient outcomes.
Conclusions
In conclusion, the excellent oncological outcomes in patients resected after TARE for intermediate/advanced HCC are confirmed, at the expense of a relatively high risk of surgical and especially biliary complications. A <6 months time from TARE to surgery is associated with a much higher risk of bile leaks. This result strongly plaids for a >6 months waiting period, taking the opportunity of selecting patients with less aggressive tumor biology, favoring FLR growth and optimizing radiation effects on the tumor.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-151/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-151/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-151/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-151/coif). F.P. and B.G. serve as the unpaid editorial board members of HepatoBiliary Surgery and Nutrition. C.A. reports consulting fees from Boston Scientific. B.G. received research grants from Roche and Guerbet and consulting fees from Roche, BMS, AstraZeneca, Canon Medical System and Boston Scientific. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional review board (IRB CHU de Montpellier 202301480) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018;68:1429-40. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Meerun MA, Allimant C, Rivière B, et al. Large, multifocal or portal vein-invading hepatocellular carcinoma (HCC) downstaged by Y90 using personalized dosimetry: safety, pathological results and outcomes after surgery. Hepatobiliary Surg Nutr 2023;12:351-65. [Crossref] [PubMed]
- Garin E, Tselikas L, Guiu B, et al. Long-Term Overall Survival After Selective Internal Radiation Therapy for Locally Advanced Hepatocellular Carcinomas: Updated Analysis of DOSISPHERE-01 Trial. J Nucl Med 2024;65:264-9. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Shehta A, Farouk A, Fouad A, et al. Post-hepatectomy liver failure after hepatic resection for hepatocellular carcinoma: a single center experience. Langenbecks Arch Surg 2021;406:87-98. [Crossref] [PubMed]
- Miller FH, Lopes Vendrami C, Gabr A, et al. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics 2021;41:1802-18. [Crossref] [PubMed]
- Gabr A, Abouchaleh N, Ali R, et al. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol 2018;29:1502-1510.e1. [Crossref] [PubMed]
- Ahmed A, Stauffer JA, LeGout JD, et al. The use of neoadjuvant lobar radioembolization prior to major hepatic resection for malignancy results in a low rate of post hepatectomy liver failure. J Gastrointest Oncol 2021;12:751-61. [Crossref] [PubMed]
- Andreatos N, Amini N, Gani F, et al. Albumin-Bilirubin Score: Predicting Short-Term Outcomes Including Bile Leak and Post-hepatectomy Liver Failure Following Hepatic Resection. J Gastrointest Surg 2017;21:238-48. [Crossref] [PubMed]
- Atassi B, Bangash AK, Lewandowski RJ, et al. Biliary sequelae following radioembolization with Yttrium-90 microspheres. J Vasc Interv Radiol 2008;19:691-7. [Crossref] [PubMed]
- Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17-29. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Khan A, Sayles HR, Dhir M. Liver resection after Y-90 radioembolization: a systematic review and meta-analysis of perioperative morbidity and mortality. HPB (Oxford) 2022;24:152-60. [Crossref] [PubMed]
- Sliwinski S, Heil J, Franz J, et al. A critical appraisal of the ISGLS definition of biliary leakage after liver resection. Langenbecks Arch Surg 2023;408:77. [Crossref] [PubMed]
- Calamia S, Barbara M, Cipolla C, et al. Risk factors for bile leakage after liver resection for neoplastic disease. Updates Surg 2022;74:1581-7. [Crossref] [PubMed]
- Mohkam K, Farges O, Vibert E, et al. Risk score to predict biliary leakage after elective liver resection. Br J Surg 2018;105:128-39. [Crossref] [PubMed]
- Sakamoto K, Tamesa T, Yukio T, et al. Risk Factors and Managements of Bile Leakage After Hepatectomy. World J Surg 2016;40:182-9. [Crossref] [PubMed]
- Braunwarth E, Primavesi F, Göbel G, et al. Is bile leakage after hepatic resection associated with impaired long-term survival? Eur J Surg Oncol 2019;45:1077-83. [Crossref] [PubMed]
- Yamashita YI, Yamamoto H, Miyata H, et al. Risk factors for bile leakage: Latest analysis of 10 102 hepatectomies for hepatocellular carcinoma from the Japanese national clinical database. J Hepatobiliary Pancreat Sci 2021;28:556-62. [Crossref] [PubMed]
- Shehta A, Farouk A, Said R, et al. Bile Leakage After Hepatic Resection for Hepatocellular Carcinoma: Does It Impact the Short- and Long-term Outcomes? J Gastrointest Surg 2022;26:2070-81. [Crossref] [PubMed]
- Aliseda D, Martí-Cruchaga P, Zozaya G, et al. Liver Resection and Transplantation Following Yttrium-90 Radioembolization for Primary Malignant Liver Tumors: A 15-Year Single-Center Experience. Cancers (Basel) 2023;15:733. [Crossref] [PubMed]
- Noda C, Williams GA, Foltz G, et al. The safety of hepatectomy after transarterial radioembolization: Single institution experience and review of the literature. J Surg Oncol 2020;122:1114-21. [Crossref] [PubMed]
- Mafeld S, Littler P, Hayhurst H, et al. Liver Resection After Selective Internal Radiation Therapy with Yttrium-90: Safety and Outcomes. J Gastrointest Cancer 2020;51:152-8. [Crossref] [PubMed]
- Yoshioka R, Saiura A, Koga R, et al. Predictive factors for bile leakage after hepatectomy: analysis of 505 consecutive patients. World J Surg 2011;35:1898-903. [Crossref] [PubMed]
- Bhattacharjya S, Puleston J, Davidson BR, et al. Outcome of early endoscopic biliary drainage in the management of bile leaks after hepatic resection. Gastrointest Endosc 2003;57:526-30. [Crossref] [PubMed]
- Sadamori H, Yagi T, Shinoura S, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg 2013;100:122-9. [Crossref] [PubMed]
- Lewandowski RJ, Donahue L, Chokechanachaisakul A, et al. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol 2016;114:99-105. [Crossref] [PubMed]
- Palard X, Edeline J, Rolland Y, et al. Dosimetric parameters predicting contralateral liver hypertrophy after unilobar radioembolization of hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 2018;45:392-401. [Crossref] [PubMed]
- Toskich B, Vidal LL, Olson MT, et al. Pathologic Response of Hepatocellular Carcinoma Treated with Yttrium-90 Glass Microsphere Radiation Segmentectomy Prior to Liver Transplantation: A Validation Study. J Vasc Interv Radiol 2021;32:518-526.e1. [Crossref] [PubMed]
- Riaz A, Kulik L, Lewandowski RJ, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology 2009;49:1185-93. [Crossref] [PubMed]
- Guiu B, Deschamps F, Aho S, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol 2012;56:609-17. [Crossref] [PubMed]
- Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 2009;20:1121-30; quiz 1131. [Crossref] [PubMed]
- Högberg J, Rizell M, Hultborn R, et al. Heterogeneity of microsphere distribution in resected liver and tumour tissue following selective intrahepatic radiotherapy. EJNMMI Res 2014;4:48. [Crossref] [PubMed]
- Pasciak AS, Abiola G, Liddell RP, et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur J Nucl Med Mol Imaging 2020;47:816-27. [Crossref] [PubMed]



