Selection of patients of hepatocellular carcinoma beyond the Milan criteria for liver transplantation
Introduction
Liver transplantation for hepatocellular carcinoma (HCC) was initially performed for lesions that were large and bilateral and thus unresectable, but recurrence of disease was common and mid-term survival was poor (1). Patients with less advanced disease had better mid-term survival (2). It was the seminal work by Mazzaferro et al. of Milan - hence known as the Milan criteria - that established an easy reference for case selection for liver transplantation for HCC. The criteria state that an HCC patient is selected for transplantation when he or she has either a single lesion not larger than 5 cm or two or three lesions not larger than 3 cm each (3). With the Milan criteria, a 4-year survival rate of 85% was achieved. It compares favorably with those achieved by transplants performed for other indications like liver failure. The Milan criteria have been adopted as a standard to justify allocations of deceased donor liver grafts from a utilitarian point of view.
The University of Southern California criteria
Although the Milan criteria provide a reliable and practical guideline for selecting HCC patients to undergo liver transplantation, they are considered rather restrictive. In order to let more HCC patients benefit from liver transplantation, Yao studied consecutive transplant recipients over a 12-year period and formulated a modest expansion of the Milan criteria: solitary HCC ≤6.5 cm, or ≤3 nodules with the largest lesion ≤4.5 cm and a total tumor diameter ≤8 cm. With this new set of criteria, a 5-year survival rate of 75% was achieved.
For this study, one should note that 76%, 16%, and 9% of the preoperative tumor staging was accurate, underestimated and overestimated respectively (4). It is also important to be aware that tumor staging in the study by Mazzaferro was done with preoperative computed tomography (3). Tested in a series by Schwartz of Mount Sinai, the expansion to the UCSF criteria offers the potential benefit of transplanting around 10% more patients with HCC without compromising survival (5).
Criteria from Asian centers
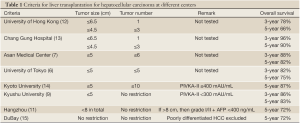
The University of Tokyo adopts the 5-5 rule: patients are selected for transplantation if they have HCC not larger than 5 cm and no more than 5 nodules. With this rule, an excellent recurrence-free survival rate of 94% was achieved (6). At Asan Medical Center, patients with HCC not larger than 5 cm and 6 or fewer nodules without gross vascular invasion are eligible for transplantation. A 5-year survival rate of 81.6% was achieved (7).
Kyoto University employed the biological marker PIVKA-II and further extended the number of HCC to 10 with the condition that serum PIVKA-II level must be lower than 400 mAU/mL. A 5-year survival rate of 86.7% was achieved (8). At Kyushu University, a 5-year survival rate of 82.7% was achieved in patients with HCC not larger than 5 cm and a serum PIVKA-II level not higher than 300 mAU/mL (9). A study in Japan involving 49 centers and 653 patients reported that patients who were beyond the Milan criteria but had serum alpha-fetoprotein levels not higher than 200 ng/mL and serum PIVKA-II levels not higher than 100 mAU/mL had a disease-free survival rate of 84.3% (10).
The Hangzhou center in China also extended the selection criteria and employed biological marker. Patients who have HCC larger than 8 cm are eligible for transplantation if their serum alpha-fetoprotein level is not higher than 400 ng/mL and their tumor biopsy shows only grade I or II differentiation. A 5-year survival rate of 72.3% was achieved (11) (Table 1).
The Toronto and up-to-7 criteria
A radical extension of inclusion criteria was proposed by the University of Toronto on the grounds of the deficiencies of the existing guidelines. It is difficult to identify small lesions accurately in multifocal HCC. Tumor size measurement may not be reproducible. Tumor behavior may not be related to tumor size and number. And overstaging (23%) or understaging (30%) of disease by imaging happens every now and again.
At the University of Toronto, HCC patients whose main lesion biopsy did not show poor differentiation were transplanted even if they had disease beyond the Milan criteria, and the 5-year survival rate of these patients was 70% while that of patients within the Milan criteria was 72% (15) (Table 1).
Tumor biopsy has the potential problem of sampling error. For instance, a nodule-in-nodule tumor can have different tumor grades (16). It has also been demonstrated that even in a single-needle biopsy, adjacent tumor cells can be of different degrees of differentiation (17). Nevertheless, if transplantation is planned for HCC beyond standard criteria, tumor biopsy appears to be a logical approach.
Biological grading of tumors by positron emission tomography has been employed to identify patients with HCC beyond the Milan criteria. However, comparable survival was found between patients beyond and patients within the criteria (18). In fact, it has been shown that HCC which is highlighted by the tracer FDG is more likely of a high grade whereas the tracer 11C-acetate used in dual-tracer positron emission tomography has a closer affinity with low-grade HCC (19). Although high-grade HCC is more likely to have microvascular invasion (20), the mere demonstration of an HCC being FDG-positive does not predict the presence of microvascular invasion (21).
Nevertheless, microvascular invasion alone does not adversely affect patient survival if the HCC is within the up-to-7 criteria, which were proposed by Mazzaferro et al. on a basis of 1556 patients from 36 centers (22,23). When the addition of the number of tumors and the size of the largest tumor (in centimeter) results in a number not larger than 7, the up-to-7 criteria are satisfied. In the study by Mazzaferro et al., patients who met the up-to-7 criteria had a 5-year survival rate of 71.2% (22).
Downstaging
Instead of extending the selection criteria, downstaging the HCC to within the Milan criteria is another logical way to transplant more patients. In a study, excellent survival was achieved after the tumors were downstaged with transarterial embolization or percutaneous ethanol injection. Of the eight patients successfully downstaged, only one patient had recurrence of HCC after liver transplantation (13).
In another study, three groups of patients were selected for downstaging using transarterial chemoembolization or laparoscopic radiofrequency ablation. The three groups were (I) patients with a single tumor ≤8 cm, (II) patients with 2 or 3 tumors each ≤5 cm and totally ≤8 cm, and (III) patients with 4 or 5 tumors each ≤3 cm and totally ≤8 cm. An observation period of three months after downstaging was mandatory. Patients were offered transplantation if they showed no tumor progression during the observation period. Forty-three of the 61 patients (70.5%) were successfully downstaged. The 35 patients who were transplanted had a 4-year survival rate of 92.1% (24). This “ablate and wait” strategy allows the tumor biology to be manifested more clearly, enabling justification of transplantation and vice versa. HCC progression after ablation is a sign of aggressive tumor behavior which warns against liver transplantation (25).
Salvage liver transplantation
It has also been proposed that patients with HCC beyond the Milan criteria can be treated initially with hepatectomy and be salvaged with liver transplantation if their recurrent HCC is within the Milan criteria and the tumors are less aggressive (26). Asan Medical Center showed that salvage liver transplantation for recurrent HCC within the Milan criteria had outcome comparable with that of primary liver transplantation (27). All too often recurrence of large and multiple HCC after hepatic resection is extrahepatic and contraindicates salvage transplantation. It has been found that around one-fifth of patients have extrahepatic recurrence and detection of recurrence may not be early enough. Moreover, salvage transplantation is applicable to less than one-third of the cases (28). Thus, Asan Medical Center proposed primary transplantation for HCC that comprises 3 or more lesions and meets the selection criteria (number ≤6 and size ≤5 cm) (7,29).
Tumor stage and characteristics at the time of salvage transplantation are closely related to tumor recurrence, especially recurrence that occurs soon after operation. HCC with a large size, multiple lesions, poor differentiation, vascular invasion and early recurrence warns against salvage transplantation.
Discussion
Hepatitis B is endemic in many parts of Asia, with a carrier rate of over 10% in the population. As hepatitis B virus is an oncologic virus for HCC, the burden of HCC is particularly heavy in these regions. Although liver transplantation is an effective treatment of HCC, the scarcity of livers donated by the deceased, which is particularly severe in Asia, has limited its application. As a result, living donor liver transplantation has become the alternative to deceased donor liver transplantation. In order to maintain a high ratio of recipient benefit to donor risk, recipient survival has to be high in living donor liver transplantation. Patients with unresectable HCC have extremely poor survival. However, a 50% post-transplant of rate is definitely better than an incurable disease. Given the inevitable donor risk, recipient survival of living donor liver transplantation for non-HCC conditions should be somewhere around 80%. However, donor enthusiasm, supply of deceased donor livers and disease burden of the region may allow some flexibility in accepting a lower recipient survival rate. But flexibility should not be abused routinely, otherwise poor recipient survival would result. It should be borne in mind that poor recipient survival means that a considerable proportion of liver donations are vain efforts.
Close auditing of donor and recipient outcomes time after time enables modification of recipient selection protocols, thereby minimizing the chance of transplanting patients with poor outlook. While published references on upper limits of tumor size and number, tumor marker level and tumor grade (Table 1) are useful in guiding clinical decision, we must also exercise judgment based on experience and scientific knowledge. A tumor with a pseudocapsule but without microvascular invasion has a small chance of extrahepatic dissemination even if it is large. Gentle handling of the tumor-housing native liver during recipient hepatectomy prevents spillage of tumor cells into the circulation and is particularly crucial when treating tumors with size beyond standard criteria.
Full table
Acknowledgements
Disclosure: None of the authors has any financial interests in or any potential conflict of interests with regard to the manuscript or its publication. None of the authors has any relationship with any company that may have a financial interest in the information contained in the manuscript. There is no financial or material sponsor for the work covered by the manuscript or for its publication. The manuscript has not been submitted or published elsewhere.
References
- Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991;110:726-34; discussion 734-5.
- Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993;218:145-51.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403.
- Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology 2004;127:S268-76.
- Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-2.
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45.
- Ito T, Takada Y, Ueda M, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637-44.
- Taketomi A, Sanefuji K, Soejima Y, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation 2009;87:531-7.
- Todo S, Furukawa H, Tada M, et al. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2007;13:S48-54.
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32.
- Chan SC, Fan ST, Lo CM, et al. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg 2008;248:411-9.
- Concejero A, Chen CL, Wang CC, et al. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation 2008;85:398-406.
- Takada Y, Uemoto S. Liver transplantation for hepatocellular carcinoma: the Kyoto experience. J Hepatobiliary Pancreat Sci 2010;17:527-32.
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72.
- Okada S, Ishii H, Nose H, et al. Intratumoral DNA heterogeneity of small hepatocellular carcinoma. Cancer 1995;75:444-50.
- Pawlik TM, Gleisner AL, Anders RA, et al. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg 2007;245:435-42.
- Kornberg A, Freesmeyer M, Bärthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592-600.
- Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med 2003;44:213-21.
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92.
- Cheung TT, Chan SC, Ho CL, et al. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl 2011;17:1218-25.
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43.
- Chan SC, Fan ST, Chok KS, et al. Survival advantage of primary liver transplantation for hepatocellular carcinoma within the up-to-7 criteria with microvascular invasion. Hepatol Int 2012.[Epub ahead of print].
- Yao FY, Kerlan RK Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008;48:819-27.
- Roberts JP, Venook A, Kerlan R, et al. Hepatocellular carcinoma: Ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925-9.
- Ikegami T, Shimada M, Imura S, et al. The timing of liver transplantation after primary hepatectomy for hepatocellular carcinoma: a special reference to recurrence pattern and Milan criteria. Transplantation 2008;86:641-6.
- Hwang S, Lee SG, Moon DB, et al. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl 2007;13:741-6.
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40.
- Hwang S, Moon DB, Lee SG. Liver transplantation and conventional surgery for advanced hepatocellular carcinoma. Transpl Int 2010;23:723-7.


