
Type IVa bile duct dilatation in an adult female treated with living donor liver transplantation
A 19-year-old female complains of an abdominal lump and intermittent pain in the upper abdomen, as well as gradually occurring skin, sclera jaundice over the past 6 months. She does not have a fever and has no complaints of bloating, nausea, or vomiting. Defecation is also normal, including the color of stool. Ten days ago, the patient experienced a high fever with a maximum body temperature of 39 ℃, accompanied by chills. This patient has no history of other illnesses or blood transfusions.
On a multislice computed tomography (MSCT), magnetic resonance cholangiopancreatography (MRCP) and biliary 3D reconstruction (Figure 1), there is evidence of intrahepatic and extrahepatic bile duct dilatation (BDD), accompanied by multiple cystic lesions in the right and left intrahepatic duct and the common bile duct but without stones. The gallbladder is also enlarged, but without signs of inflammation. Anatomical abnormalities or thrombosis in the hepatic artery, portal vein, or hepatic vein cannot be seen. The pancreas, kidneys (bilateral), urinary bladder, and rectum show no abnormalities. The confluence of the bile duct and pancreatic duct is not well identified on computed tomography (CT). Obstructive jaundice and abnormal coagulation function are indicated by laboratory testing of total bilirubin 273.3 µmol/L, direct bilirubin 237.3 µmol/L, and prothrombin time 30.1 sec. The amylase content in bile drainage fluid is 70,815 U/L. Thus, the final diagnosis is made with type IVa BDD with obstructive jaundice.
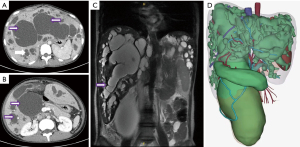
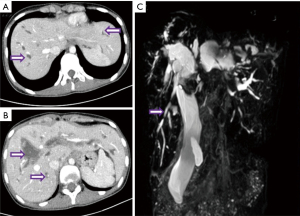
BDD is a clinically rare primary biliary tract disease, mainly characterized by single or multiple local dilations of the intrahepatic and/or extrahepatic bile ducts (1). According to the standard Todani classification, BDD is classified into 5 types (2). Type IVa BDD is the multifocal dilation of both intrahepatic and extrahepatic bile ducts (3). The most common cyst-related complication is recurrent cholangitis; in addition, BDD is thought to be a premalignant condition (4,5). Therefore, curative surgery should completely remove the dilated bile ducts both extrahepatically and intrahepatically. We have previously reported aggressive hepatectomy for selected patients with bilobar BDD of type IVa, whose bilobar BDD was considered as localized, with the volume of remaining healthy segments more than 30% of standard liver volume (6). For this patient, based on the imaging examination at admission (Figure 1), we believe she has the more challenging diffuse type of dilation, where the dilated bile ducts involve all liver segments. How to provide appropriate treatment for this patient has become a challenge. We observe that the dilation of her extrahepatic bile ducts is also exceptionally severe (Figure 1B,1D). So, is the dilation of her intrahepatic bile duct all pathological cystic dilation? Or are there also some “columnar dilation” secondary to obstruction of the extrahepatic bile duct at the same time? We then perform percutaneous transhepatic biliary drainage (PTBD) on the patient for further diagnosis and simultaneous relief of symptoms of obstructive jaundice and cholangitis. After one month of drainage, the patient’s jaundice symptoms improve significantly (total bilirubin 66 µmol/L, direct bilirubin 56.9 µmol/L). CT and MRCP results indicate that intrahepatic and extrahepatic bile duct dilation still exists (Figure 2). Especially for intrahepatic dilated bile ducts, after undergoing biliary decompression, obvious abnormal bead-like cystic dilation can still be seen at the level of the liver segment. After multidisciplinary discussions, we decided to adopt the treatment method of living donor liver transplantation. At the same time, we also realize that completely removing the diseased bile duct of the pancreatic segment while avoiding pancreatic duct damage is also one of the difficulties in surgery.
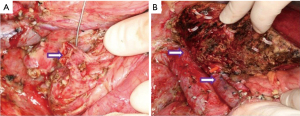
After sufficient preoperative evaluation, we obtain the left liver of the patient’s mother without the middle hepatic vein as a graft [379 g, graft-to-recipient weight ratio (GRWR) =0.98%]. After laparotomy, we find that the liver is dark brown with cholestasis, significantly enlarged, and hard in texture. A cystic mass with a size of approximately 8 cm × 9 cm can be seen on the right edge of the hepatoduodenal ligament, with a smooth surface but adhered to surrounding tissues. Its lower end is tightly adhered to pancreatic tissue. Follow the surgical procedure of liver transplantation to free the diseased liver and disconnect the corresponding tubes. For the extrahepatic bile duct cyst, we carefully separate it into the pancreatic segment of the bile duct, sever the bile duct at a distance of about 1 cm from the pancreatic duct at the confluence of the bile duct and pancreas, and lock it (Figure 3A). When implanting the new liver, we cut open the common trunk of the left hepatic vein of the recipient and moderately extend it towards the direction of the vena cava to enlarge the shape by about 3.8 cm, making it match the size of the outflow tract opening after the donor liver plastic surgery. We reconstruct the hepatic vein outflow tract using a 5/0 Prolene suture end-to-end continuous suture method. Subsequently, the portal vein is anastomosed end-to-end using a 6-0 Prolene suture, and the hepatic artery is reconstructed by end-to-end suturing with a 9-0 Prolene suture (Figure 3B). Finally, Roux-en-Y cholangiojejunostomy is performed to restore biliary patency. The entire surgery takes 825 minutes, with intraoperative bleeding of 200 mL. Postoperatively, the patient had a favorable prognosis without significant transplant-related complications, including acute rejection, biliary leak, hepatic artery/portal vein stenosis or thrombosis, or outflow obstruction. Notably, the patient developed a grade B pancreatic leak, which resolved completely after approximately one month of drainage therapy.
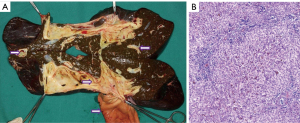
By examining the pathological liver in the sagittal plane, the dilated intrahepatic and extrahepatic bile ducts are clearly visible. The pathological dilation of intrahepatic bile ducts progresses to the level of liver segments. The liver exhibits feature of cholestatic cirrhosis as a whole (Figure 4A). Histopathological examination reveals expansion of the portal tracts with fibrosis, and adjacent tracts are connected to form septa that separate the surrounding hepatic parenchyma, consistent with biliary fibrosis. Additionally, there is an increase in the number of interlobular bile ducts and small bile ducts within the portal tracts, which is indicative of large duct disease (Figure 4B, HE 10×).
This patient provides us with new experiences and insights in treating type IVa BDD, where the dilation of intrahepatic bile ducts extends throughout the entire liver, affecting the third-order bile ducts. Thanks to the advancement of liver transplantation technology, particularly the maturation of living donor liver transplantation, we now have a wider and better array of options for treating complex benign biliary diseases. For patients with BDD, complete resection of the affected biliary tract is essential to help them completely avoid the risks of recurrent cholangitis, biliary calculi, and even biliary tract cancer. Careful preoperative analysis of imaging studies is essential for delineating the scope and severity of biliary dilation and for ascertaining whether intrahepatic biliary dilation is a consequence of extrahepatic biliary stenosis, which are critical factors in formulating the surgical strategy.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-563/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-563/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ciccioli C, Mazza S, Sorge A, et al. Diagnosis and Treatment of Choledochal Cysts: A Comprehensive Review with a Focus on Choledochocele. Dig Dis Sci 2025;70:39-48. [Crossref] [PubMed]
- Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977;134:263-9. [Crossref] [PubMed]
- Brown ZJ, Baghdadi A, Kamel I, et al. Diagnosis and management of choledochal cysts. HPB (Oxford) 2023;25:14-25. [Crossref] [PubMed]
- Anand U, John AG, Priyadarshi RN, et al. Long-term complications after extrahepatic cyst excision for type IV-A choledochal cysts. Ann Hepatobiliary Pancreat Surg 2023;27:433-6. [Crossref] [PubMed]
- Takeshita N, Ota T, Yamamoto M. Forty-year experience with flow-diversion surgery for patients with congenital choledochal cysts with pancreaticobiliary maljunction at a single institution. Ann Surg 2011;254:1050-3. [Crossref] [PubMed]
- Dong JH, Yang SZ, Xia HT, et al. Aggressive hepatectomy for the curative treatment of bilobar involvement of type IV-A bile duct cyst. Ann Surg 2013;258:122-8. [Crossref] [PubMed]


