Effectiveness of additional resection of the invasive cancer-positive proximal bile duct margin in cases of hilar cholangiocarcinoma
Introduction
Hilar cholangiocarcinoma (HCCA), or Klatskin tumor, is an advanced tumor at or near the confluence of the right and left hepatic duct. Radical surgical resection (extrahepatic bile duct resection, hepatectomy with en-bloc total caudate lobe resection, and regional lymphadenectomy along with (+/−) vascular resection and reconstruction) is the only way to get long-term survival and potential cure (1,2). A tumor-free resection margin (R0) is a critical factor for survival and is the only factor that can be modified surgically (2). The remaining microscopic invasive carcinoma at the ductal resection margin leads a poor survival for the patients (3,4). Contrastingly, cases with complete resection have 5-year survival rates of 25–40% (5). Therefore, R0 resection could improve surgical outcomes for patients with HCCA. Microscopic tumor spread along the bile ducts beyond the gross tumor border is a characteristic feature of this tumor, inducing unlooked-for tumor infiltration of the resection margin (2). Therefore, a wide enough R0 margins are required for curative resection. More extensive resection is recommended to achieve R0 resection when a positive proximal bile duct resection margin [PM(+)] is demonstrated on intraoperative frozen section (FS) analysis. However, this is often difficult because of invasion onto critical vessels and adjacent liver parenchyma, and additional resection of the liver parenchyma often increases the morbidity and mortality. It is not technically possible sometime.
Serum carbohydrate antigen 19-9 (CA19-9) has been used extensively in clinical treatment process of HCCA. Additional resection of the PM(+) might be restricted only in patients with low levels of CA19-9 and no distant metastasis (6). Previously, we reported that preoperative CA 19-9 levels predict resectability, survival, and early recurrence in patients with resectable HCCA (7,8). Thus, tumor markers also partially represent the degree of malignancy of the tumor.
Studies of intraoperative additional resection of the PM(+) HCCA produced conflicting conclusions (1,6,9-13). Some descripted that additional resection achieved a significant survival benefit; whereas others did not (1,9,10). Thus, this study designed to investigate the effectiveness of additional resection of PM(+) compared with negative proximal bile ductal margin without additional resection, considering the level of the preoperative CA19-9.
Methods
Patients
Five hundred and twenty-seven patients diagnosed with HCCA at West China Hospital, Sichuan University between June 2000 and January 2017 were identified from a retrospectively collected database. Patients who have undergone curative resection of histologically-proven HCCA were included. Patients with preoperative serum CA19-9 levels <5 U/mL (CA19-9 non-secretory), and cholangitis were excluded. Only patient who had undergone the extrahepatic bile duct resection plus hepatectomy and regional lymphadenectomy were enrolled. Informed consent was obtained from all patients for surgical treatment. Data collection and analysis were performed according to the ethical standards of the Helsinki Declaration.
Preoperative evaluation and surgery
We previously described our standard management of HCCA (7,8,14). Multi-detector row spiral computed tomography (MDCT), and magnetic resonance imaging (MRI) were used to evaluate the location, extent, and staging of the tumor. Preoperative biliary drainage was used for preoperative optimization of the liver, using endoscopic retrograde cholangiopancreatography (ERCP) (n=46) and percutaneous transhepatic cholangiodrainage (PTCD) (n=128). The optimal cut-off value for preoperative CA19-9 was set at 150 U/L which was reported by our previously study (8). Unresectable patients were identified as those with: (I) advanced bile duct infiltration that precluded intact tumor removal; (II) invasion of major vascular systems, such as bilateral portal vein involvement, which hampers vascular reconstruction; (III) lymph nodes metastases beyond the hepatoduodenal ligament; (IV) unilateral hepatic lobe atrophy with invasion of the contralateral portal vein or hepatic artery; (V) unilateral hepatic lobe atrophy with invasion of the contralateral secondary biliary radicles; (VI) unilateral secondary biliary radicles involvement with invasion of the contralateral portal vein or hepatic artery; and (VII) pathologically confirmed HCCA with evidence of distant metastases (7). The volumes of liver to be resected were calculated using serial MDCT images. Selective preoperative portal vein embolization was indicated to decrease the risk of postoperative hepatic failure in patients whose residual liver parenchymal volume was less than 40% of the total liver volume. The preoperative serum CA19-9 level (pr-CA) was assayed immediately before surgery because it can be affected by cholestasis or cholangitis.
Pathological evaluation and measurement of the length of proximal ductal margin (PM)
Proximal bile ducts were transected above the level of the gross tumor and regarded as the specimen margin (Figure 1) (9). The specimen margin was not submitted for FS analysis. A separate cut-off of the proximal bile duct (ductal margin) above the initial specimen margin (toward the liver remnant) was then transected and submitted for FS analysis (Figure 1). When the PM was positive for invasive carcinoma, additional resection was adopted to achieve a R0 margin, if technically possible. A cut-off from the distal common bile duct margin was also submitted for FS analysis in each case. When the distal ductal margin (DM) was positive, additional resection, including combined pancreaticoduodenectomy, if needed, was performed. All margins submitted for intraoperative FS analysis were subsequently examined histopathologically.
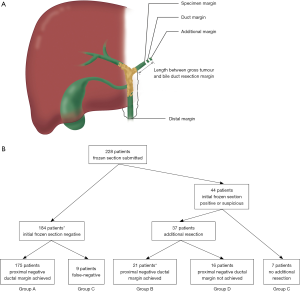
All histological sections were reviewed by two experienced pathologists who were blinded to the clinical information. The ductal margin status, and the length between the gross tumor and the proximal bile duct resection margin [length of the PM (LPM)] were assessed histologically and calculated based on the permanent FS and formalin-fixed resected specimens (Figure 1). Patients were divided into four groups regarding whether intraoperative further resection of the positive PM was performed and the condition of the proximal PM: group A, PM(−) without additional resection; group B, PM(−) with additional resection; group C, final PM(+) without additional resection; and group D, final PM(+) with additional resection. R0 resections were defined as resection without microscopic tumor cells detected in the biliary, vascular, or hepatic parenchymal surgical margins. R1 resections were defined as microscopic tumor deposits in one of the above-mentioned surgical margins. R2 resections were defined as those with a macroscopic tumor left behind in one or more of the surgical margins. Palliative therapy comprised palliative biliary drainage, chemotherapy, or radiotherapy. Nodal status was positive (N1) if one or more of the hilar lymph nodes was infiltrated with tumor cells. N1 comprised 1–3 regional lymph node metastases; and N2 comprised ≥4 regional lymph node metastases. Patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system for HCCA (15).
Follow-up
HCCA is a disastrous malignant disease; therefore, all patients were strictly supervised and followed-up in outpatient clinics. The enrolled patients were assessed every 2–3 months by assessment of liver function, tumor markers, and ultrasonography in the first year after surgery, and thereafter at 3–6 months annually. If recurrence or distant metastasis was suspected, computed tomography or MRI was conducted. The date of the first suspicious radiological finding represented the date of initial disease recurrence.
Statistics
Variables are presented as absolute numbers, percentage or median values, and ranges. Statistical analysis comprised nonparametric tests using the Mann-Whitney U test, Chi-square test, or Fisher’s exact test, when appropriate. Univariate analysis of survival probabilities was estimated using the Kaplan-Meier log rank test, from the time of operation to the time of death or the most recent follow-up, excluding postoperative deaths (any deaths occurring within 90 days of surgery or during the same hospital stay, whenever it occurred). All data were updated on July 1, 2017. Factors with P<0.20 in the univariate analysis were subjected to multivariate analysis using the Cox proportional-hazards model, which was also used to evaluate the interactions between the prognostic factors and the LPM on overall survival. Statistical significance was set at P<0.05. The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 24 (IBM Corporation, Armonk, NY, USA).
Results
Between June 2000 and January 2017, we treated 527 patients with HCCA in our institution, of whom 228 patients were enrolled in the study. Group A: 175 patients, group B: 21 patients, group C: 16 patients, and group D: 16 patients.
Comparison of group A, B, C and D
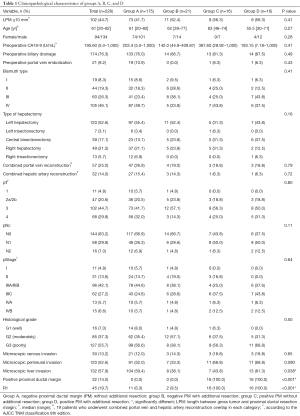
The clinicopathological characteristics of the four groups is presented in Table 1. There were significantly more patients with microscopic liver invasion in group D compared with those in groups A, B, and C (P=0.038, Table 1). Rates of combined portal vein and/or hepatic artery resection were similar among all groups (P>0.05, Table 1).
Full table
Among the patients, 184 had an initial negative PM, and additional resection was not performed in nine patients from this group because of false-negative diagnosis on FS; therefore, they were excluded from group A. Of the 44 patients who were initially PM(+), 37 underwent additional resection bringing about 21 PM(−) cases (group B) and 16 final PM(+) patients (group D). Additional resection was not performed in seven patients because of their unfit general condition and long operation time. Ultimately, 32 (9+16+7) (14%) of the 228 patients were PM(+), without (group C, n=16) or with additional resection (group D, n=16).
The type of hepatectomy was similar among the four groups (P=0.16). In left hepatectomy (hemihepatectomy + trisectionectomy), 24 patients were initially PM(+). Among them, 11 (45.8%) became PM(−) after additional resection. In contrast, in right hepatectomy, 13 patients were initially PM(+). Among them, 5 (38.5%) became PM(−) after additional resection. The difference was not significantly different (P=0.74).
For final resection margin status (R), the rate of R1 resection was 6.3% (11/175) in group A, 9.5% (2/21) in group B, 100% (16/16) in group C, and 100% (16/16) in group D. Significantly more R1 resections were performed in groups C and D than in groups A and B (P<0.001). Accordingly, 183 patients (80.3%) had R0 resection, whereas 45 R1 resection patients (19.7%).
The length between the gross tumor and the proximal ductal resection margin (LPM), age, sex, pr-CA, preoperative biliary drainage, preoperative portal vein embolization, pTNM Stage, histological differentiation, and microscopic venous invasion were not statistically different between the groups (all P>0.05). In all patients, pr-CA was positively correlated with the total bilirubin level (r=0.234, P=0.000).
Relationship between local recurrence rate and ductal margin status
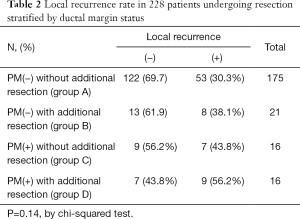
Local recurrence was found in 77 of the 228 patients (33.8%). When local recurrence was investigated according to the ductal margin status, local recurrence was observed in 53 patients (30.3%) in group A, in 8 patients (38.1%) in group B, in 7 patients (43.8%) in group C, and in 9 patients (56.2%) in group D. There was no significant relationship between local recurrence and ductal margin status (P=0.14, chis squared test, Table 2). However, an improving trend was found for the rate of local recurrence when a negative proximal bile duct resection margin could not be achieved by additional resection (Table 2).
Full table
Survival and prognostic factors
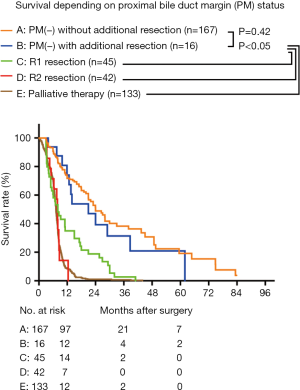
The median survival rates of PM(−) without additional resection, PM(−) with additional resection, R1 resection, R2 resection, and palliative therapy were 23.98 (95% CI, 19.09–28.88) months, 20.99 (95% CI, 6.67–35.31) months, 10.47 (95% CI, 8.76–12.18) months, 7.87 (95% CI, 7.49–8.25) months, and 7.23 (95% CI, 6.94–7.53) months, respectively. The difference between PM(−) without additional resection and PM(−) with additional resection under R0 resection was not significant (P=0.42). The median survival of R0 resection was significantly better than that of R1 resection, R2 resection, and palliative therapy (P<0.05). The median survival of R1 resection was also significantly better than that of R2 resection and palliative therapy (P<0.05). The survival rates of R2 resection and palliative therapy were similar (P=0.98) (Figure 2).
The overall survival rate of the 228 patients was 64.7% at 1 year, 28.7% at 3 years, and 13.9% at 5 years. The median survival was 20.98 (95% CI, 16.98–24.98) months, and the median follow-up time was 25.99 (3.02–88.7) months.
On univariate analysis, 10 of the 17 possible clinicopathological prognostic factors, including preoperative CA19-9, proximal additional resection, LPM, Bismuth type, tumor stage (pT), lymph nodes metastases, the TNM stage (pStage), histological grade, microscopic liver invasion, and resection margin status (R) were significant (Table 3). Multivariate analysis of the 10 significant factors demonstrated that LPM [hazard ratio (HR): 1.96], pT (HR: 0.18), and resection margin status (HR: 0.36) were independent prognostic factors for survival (Table 3).
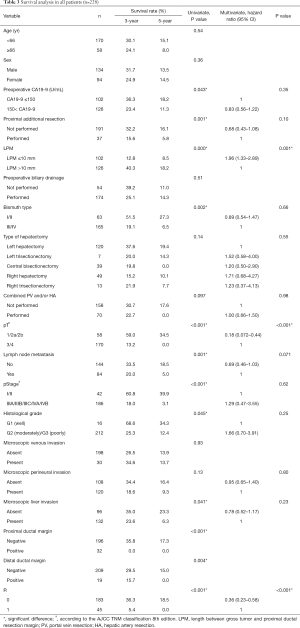
Full table
Interactions among CA19-9, additional resection of the PM, LPM, and survival
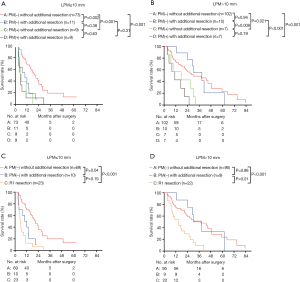
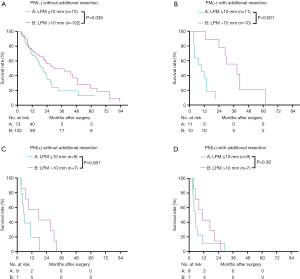
We then analyzed the overall survival according to the final PM status, using groups A–D. The 1- and 3-year survival of groups A, B, C, and D were 70.9%, 37.0%, 66.0%, and 28.6%; and 38.1%, 0.0%, and 25.0%, and 0.0%, respectively (Figure 3A). The median survival of groups A–D were 23.98 (95% CI, 19.09–28.88) months, 20.99 (95% CI, 6.67–35.31) months, 11.60 (8.22–14.98) months, and 9.50 (7.07–11.92) months, respectively. The overall survival of groups A and B were similar (P=0.16), and both were significantly higher than those of groups C and D (P<0.05). The overall survival of groups C and D were similar (P=0.23) (Figure 3A). To better compare the overall survival of R1 resection and each PM(−) group, we separated the R1 resection individually. After separating R1 individually, the 3-year survival and median survival in groups A and B remained similar and were both significantly better than that in the R1 resection group (P<0.05, Figure 3B). The median survival of LPM >10 mm [27.99 (95% CI, 22.54–33.45) months] was significantly better than that of LPM ≤10 mm [15.01 (95% CI, 10.20–19.83) months] (P<0.001, Table 2).
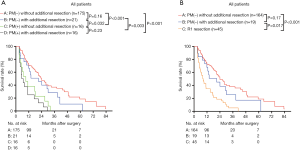
Next, the survival rates of the four groups were compared in subgroups stratified according to the pr-CA value [cut-off value =150 U/L (8)], The patients were divided into pr-CA >150.0 U/mL (pr-CA high (H)) and pre-CA ≤150.0 U/mL [pr-CA low (L)] subgroups. Figure 4A,B shows that the overall survival of group B was similar to that of group A in the two pre-CA subgroups (P>0.05), even after separating R1 resection individually (P>0.05, Figure 4C,D). For pr-CA19-9≤150.0 U/mL and pre-CA19-9>150.0 U/mL, the survival of groups A and B were significantly better than those of group D (both P<0.05). After separating R1 resection individually, in all pr-CA subgroups, the survival of group A and B was similar (P>0.05), and both were significantly better than that of R1 resection (P<0.05) (Figure 4C,D).
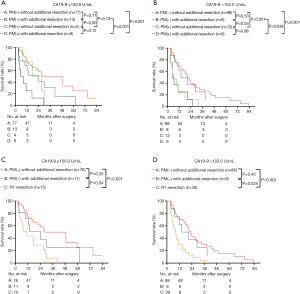
Figure 5 presents the survival of the enrolled patients stratified by LPM and R1 resection for different levels of pre-CA19-9: LPM ≤10 mm, and LPM >10 mm. The median survival of patients with LPM >10 mm [35.01 (95% CI, 26.54–49.49) months] was significantly better than that of patients with LPM ≤10 mm [19.06 (95% CI, 14.45–23.66) months] and R1 resection [10.47 (95% CI, 8.76–12.18) months] (Figure 5A). For pre-CA19-9 ≤150 U/mL, the survival of patients with LPM ≤10 mm and LPM>10 mm was similar (P=0.14) and were significantly better than that of R1 resection (P<0.05, Figure 5B). For pre-CA19-9 >150 U/mL, the survival of patients with LPM >10 mm was significantly better than that of patients with LPM ≤10 mm (P=0.011) and R1 resection (P<0.001). The survival of patients with LPM ≤10 mm was also significantly better than that of R1 resection (P=0.005).
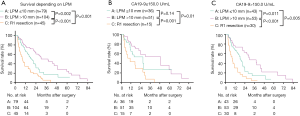
Figure 6A,B shows the survival of the four groups stratified by LPM. In the LPM ≤10 mm, the median survival of group A [20.96 (95% CI, 15.65–26.27) months] was significantly better than that of group B [9.86 (95% CI, 3.13–16.59) months, P=0.002], C [5.99 (95% CI, 3.57–8.42) months, P<0.001], and D [5.03 (95% CI, 2.12–7.94) months, P<0.001]. The survival of group B, C and D was similar (Figure 6A). In the LPM >10 mm subgroup, the median survival of group B [28.70 (95% CI, 17.54–39.87) months] was comparable with that of group A [29.96 (95% CI, 13.17–46.76) months] (P=0.94), and the survival of both these groups was significantly better than those of groups C [17.00 (95% CI, 0.00–38.80) months] and D [11.00 (95% CI, 3.36–18.65) months] (P<0.05). The survival of group C and D was similar (P=0.19) (Figure 6B). After separating R1 resection individually, in the LPM ≤10 mm subgroup, the median survival of group A [20.96 (95% CI, 15.65–26.27) months] remained significantly better than that of group B [9.86 (95% CI, 3.13–16.59) months, P=0.04] and R1 resection [5.50 (95% CI, 3.79–7.20) months, P=0.00], but there was no significant different between the survival of group B and R1 resection (P=0.19, Figure 6C). In the LPM >10 mm subgroup, the median survival of group B [30.03 (95% CI, 18.52–41.54) months] was comparable with that of group A [35.01 (95% CI, 20.35–55.68) months] (P=0.98), and the survival of both these groups was significantly better than that of R1 resection [15.0 (95% CI, 6.77–23.23) months] (P<0.05, Figure 6D). The survival of patients with LPM >10 mm was significantly better than those with LPM ≤10 mm (P<0.05, Figure 7A,B) except for the PM(+) patients (P>0.05, Figure 7C,D).
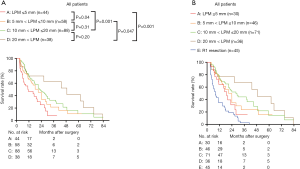
To further investigate the effect of LPM on overall survival, we further divided the patients into groups regarding the LPM: LPM ≤5 mm: 44 patients; 5 mm < LPM ≤10 mm: 58 patients; 10 mm < LPM ≤20 mm: 88 patients; 20 mm < LPM: 38 patients. We found that The OS of LPM ≤5 mm (median: 11.02 months) was significantly worse than that of other groups (P<0.05, Figure 8A). The OS of 5 mm < LPM ≤10 mm was significantly worse than that of 20 mm < LPM (P=0.047, Figure 8A). The OS of 10 mm < LPM ≤20 mm and 20 mm < LPM was not significantly different (P=0.20, Figure 8A,B).
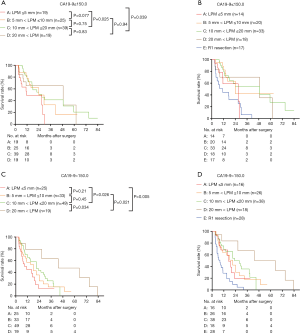
Then the survival rates of the four groups were compared in the subgroups stratified according to the value of preoperative CA19-9. When pre-CA19-9 ≤150.0 U/mL, the overall survival of LPM ≤5 mm was significantly worse than that of 10 mm < LPM ≤20 mm and 20 mm < LPM (Figure 9A), but not significantly differ from that of R1 resection (P>0.05, Figure 9B). The overall survival of 5 mm < LPM ≤10 mm, 10 mm < LPM ≤20 mm, and 20 mm < LPM were significantly better than R1 resection (Figure 9B); When pre-CA19-9>150.0 U/mL, the and overall survival of 20 mm < LPM was significantly better than that of LPM ≤5 mm, 5 mm < LPM ≤10 mm, 10 mm < LPM ≤20 mm, and R1 resection (Figure 9C,D).
Discussion
Although complete tumor resection on histological examination (R0) is the most meaningful factor influence long-term prognosis in the surgical treatment of HCCA (2), several studies have advocated that long-term survival after R0 and R1 was not significantly different (11,16,17). In the present study, the survival of R1 resection was significantly worse than R0 resection, but still better than R2 resection and palliative therapy. This is consistent with some previous reports in which the survival rates of R1 resection patients were significantly better than those with unresectable tumors (18-20).
Our findings indicated that a PM(−) achieved by further resection resulted in superior survival than a positive PM without additional resection cases. The prognostic significance of further resection of a PM(+) margin in HCCA is disputed. Although a significant survival benefit of further resection was reported by Ribero et al.. Endo et al. and Shingu et al. reported that additional resection did not improve survival (1,9,10). Oguro et al. presented that only patients with a lower level of CA19-9 and no distant metastasis could benefit from a final PM(−) achieved by further resection (6). In the present study, the survival of the 21 PM(−) patients resulting from additional resection did not differ significantly differ from 175 PM(−) patients without additional resection and was significantly better compared with all PM(+) or R1 resection patients. Oguro et al. thought that the effectiveness of further PM resection in bettering survival is affiliated with the degree of cancer progression, and the discrepancies in the conclusions of previous studies might be illustrated by differences in the tumor characteristics of the enrolled populations. In their report, 40% of cases were Bismuth IV disease, one of the most advanced and longitudinal wide spreading perihilar cholangiocarcinomas, which was similar to that of Shingu et al. (38.9%). The proportion was only 14.6% in the study by Ribero et al. Therefore, Oguro et al. concluded that patients with less advanced tumors might show better survival after re-resection of the positive PM. However, the proportion of Bismuth IV disease was 0% in the study by Endo et al. The proportion of Bismuth IV cases was a relatively high 46.9% in our study. Endo et al. and Shingu et al. reported that the survival of patients with a short proximal negative ductal margin achieved by additional resection was worse than that for patients who underwent an R0 resection with a longer ductal margin and was similar to that for patients who underwent an R1 resection. However, Ribero et al. and Oguro et al. did not investigate the width of their proximal bile ductal margin. It is possible that the width of the PM could explain the discrepancy in the results of among the studies. In our study, we found that The OS of LPM ≤5 mm was significantly worse than that of other groups (Figure 8A). The OS of 5 mm < LPM ≤10 mm was significantly worse than that of 20 mm < LPM (P=0.047, Figure 8A). The OS of 10 mm < LPM ≤20 mm and 20 mm < LPM was not significantly different (P=0.20, Figure 8A). Therefore, a wider proximal bile ductal margin could help to increase the overall survival.
In our study, 16 PM(+) patients who underwent additional resection did not achieve a negative proximal DM, which may be attributed to a false-negative diagnosis on intra-operative FS analysis. Generally, the intraoperative status of the ductal margin is assessed using histopathological FS analysis (9). However, the inaccuracy of FS analysis to determine the presence of invasive carcinoma or epithelial atypia at the bile duct margin should be considered when compared with permanent histopathological analysis, especially after biliary drainage procedures (9). The sensitivity of intraoperative FS was reported as 68–75% (13,21). The inconsistency between FS and permanent histopathological analyses of bile ductal margin may explained by several factors. Inflammatory stromal infiltration of the tumor into the surrounding duct is an inherent characteristic of cholangiocarcinoma (22). the mistaken margin assessment of intraoperative FS analysis may attributed to the presence of atypical cells within the boundary zone between the tumor and the normal duct epithelium, and the propensity for submucosal tumor extension (9).
The pre-CA19-9 level has been used as a useful prognostic marker in patients with gastrointestinal cancers (23-25). Unfortunately, only a few studies have reported the prognostic value of the pre-CA19-9 level in patients with biliary carcinomas. As coexisted obstructive jaundice is generally a feature of HCCA, which has an impact on the serum CA19-9 level. It is complicated to clarify the effect of an elevated CA19-9 level is (26-29). Thus, assessing the pre-CA19-9 level after suitable biliary drainage is recommended to predict long-term survival. The CA19-9 level might be a significant prognostic factor only in limited patients with resected HCCA (6) Previously, we reported that preoperative CA 19‐9 (>150 U/mL) levels is associated with poor resectability, poor survival, and higher tendency for early recurrence (7,8). We employed the pr-CA19-9 value in present study population to investigate its prognostic value on the proximal tumor-free margin. Regardless of the pr-CA19-9 level, the survival of group A and B patients were similar. The survival of patients in group B was significantly higher than that of group D (P<0.05), but not significantly different with that of patients in group C. The survival effectiveness of further resection of a positive PM might be associated with the value of pre-CA19-9. Individuals with a Lewisa-b phenotype (lacking the Lewis antigen glycosyl-transferase) are unable to synthesize CA19-9 (30). Approximately 10% of the Japanese population are Lewisa-b and these individuals do not express CA19-9 at all (31). Consequently, we excluded patients with pr-CA <5.0 U/mL to avoid false negatives.
To better compare the effects of different LPMs on survival, we divided the enrolled patients into LPM ≤10 mm and LPM >10 mm subgroups. The results supported the view that a wider proximal bile duct margin could achieve better overall survival. The patients with LPM>10 mm survived longer than patients with LPM ≤10 mm especially those with pr-CA19-9 >150 U/mL. Further resection of the PM(+) margin was operated to secure a clear margin whenever technically possible. Ebata et al. suggested that a 10-mm, even a 20-mm margin is required to eradicate invasive bile duct carcinoma (32). Seyama et al. showed that the survival of patients with surgical tumor-free margin >5 mm was significantly better than that of patients with a margin <5 mm. However, the survival of patients after R0 resection did not significantly differ from those of patients with a narrow margin (<5 mm) or received R1 resection (16). Sakamoto et al. suggested a 5-mm tumor-free margin due to anastomotic recurrences never happened if a proximal tumor-free resection margin >5 mm was achieved (33). Additionally, the proximal longitudinal invasion of HCCA tumors range from 0.6 to 18.8 mm in the submucosal layer (2). These reports suggested that the resection margin status should be redefined when a proximal tumor-free resection margin <5 mm, and an LPM >10 mm is recommended to achieve significantly better survival. The different survival results between studies by Ribero et al., Endo et al., Shingu et al. and Oguro et al. might be attributed to the different LPMs. However, the LPM was not presented or investigated in previous studies on additional resection (1,6,9-11,13). In our study, the survival of patients with LPM ≤10 mm was significant worse than that of patients with LP >10 mm (P<0.05), which is an independent prognostic factor for survival. With an LPM ≤10 mm, there was no significant difference among the survival rates of groups B, C, and D, or between group B and R1 resection after separating R1 resection individually. The survival of LPM>10 mm patients was significantly better than LPM ≤10 mm patients (P<0.05) in group A and B. However, the survival of LPM>10 mm and LPM ≤10 mm patients were similar in group C and D. To further investigate the effect of different LPM on overall survival, we compared the survival of different LPM according to the value of preoperative CA19-9. When pre-CA19-9 ≤150.0 U/mL, the overall survival of LPM ≤5 mm was significantly worse than that of 10 mm < LPM ≤20 mm and 20 mm < LPM, but not significantly differ from that of R1 resection. The overall survival of 5 mm < LPM ≤10 mm, 10 mm < LPM ≤20 mm, and 20 mm < LPM were significantly better than R1 resection; When pre-CA19-9 >150.0 U/mL, the overall survival of 20 mm < LPM was significantly better than that of LPM ≤5 mm, 5 mm < LPM ≤10 mm, 10 mm < LPM ≤20 mm, and R1 resection. The above findings show that, for R0 resection, the pr-CA19-9 level is more associated with the LPM, rather than with whether additional resection is performed when a positive proximal margin is found intraoperatively. When pre-CA19-9 ≤150.0 U/mL, the proximal margin wider than 10 mm potentially achieve a better survival benefits; When pre-CA19-9 >150.0 U/L, the LPM wider than 20 mm may achieve a significant better survival benefit.
It is technically difficult to perform further resection at the proximal margin in PM(+) patients as only a few millimeters of bile duct can be respected. Addition resection of more than 1 cm is difficult in most patients (10). In our study, 47.6% (10/21) patients received additional resection >1 cm. Additional resection of more than 0.5–1 cm of the proximal bile duct means additional resection of a liver segment, or that additional choledochojejunostomy is needed, which significantly increases the damage to the patients. Therefore, it is necessary to consider the patient’s general condition comprehensively when performing an additional resection.
Most cases of HCCA are encountered after invasion into the periductal connective tissue or surrounding liver (34). It is commonly to encounter that the periductal hilar tissues and adjacent liver tissue were directly invaded, even in well-differentiated adenocarcinoma. Somer et al. identified that the tumor had a very prominent, direct extension into the liver (35). Once a tumor infiltrates beyond the serum, perineural invasion could be seen in 81.4% cases (36), and vascular structures are also often invaded by tumors. Hepatic invasion could be observed in up to 76% of HCC patients under surgery (37). The mean distance of microscopic invasion beyond the gross margin toward the liver is 16.8 mm, making it difficult to evaluate and obtain an R0 resection (38). This was supported by the present study, in which the rate of microscopic liver invasion was significantly higher in group D.
There are several limitations in the current study. First, the sample size was small, especially the additional resection and PM(+) groups, because it often depends on the accuracy, sensitivity, and specificity of intraoperative FS to decide whether additional resection should be performed. The invasion of critical vessels and adjacent liver parenchyma of HCCA also increases the difficulty of additional resection to achieve R0 status. Second, this study was drawn from a single geographical area. Third, HCCA is generally diagnosed at an advanced stage and many patients have hyperbilirubinemia. The pr-CA is always associated with increased total bilirubin level, which affects the results analysis. Thus, further research and multicenter studies should be carried out to support the clinical utility of our findings.
In conclusion, the survival benefit of further resection of the positive proximal margin in HCCA was not significantly different to negative proximal margin without additional resection. It has a limited association with the pr-CA19-9 level. An LPM ≥10 mm is potentially more associated with the survival benefit compared with additional resection of the positive proximal margin, when performed under different pr-CA19-9 levels.
Acknowledgements
Wen-Jie Ma wants to thank, in particular, the invaluable support received from C.B. Hu over the years.
Funding: We acknowledge the Science & Technology Support Project of Sichuan Province (Nos. 2018JY0019, 2015FZ0076, and 2014SZ0191).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the ethics committee review board of Sichuan University. Informed consent was obtained from all patients for surgical treatment. Data collection and analysis were performed according to the ethical standards of the Helsinki Declaration.
References
- Ribero D, Amisano M, Lo Tesoriere R, et al. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg 2011;254:776-81; discussion 781-3. [Crossref] [PubMed]
- Xiang S, Lau WY, Chen XP. Hilar cholangiocarcinoma: controversies on the extent of surgical resection aiming at cure. Int J Colorectal Dis 2015;30:159-71. [Crossref] [PubMed]
- Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672-9. [Crossref] [PubMed]
- Kang MJ, Jang JY, Chang J, et al. Actual Long-Term Survival Outcome of 403 Consecutive Patients with Hilar Cholangiocarcinoma. World J Surg 2016;40:2451-9. [Crossref] [PubMed]
- Ito F, Cho CS, Rikkers LF, et al. Hilar cholangiocarcinoma: current management. Ann Surg 2009;250:210-8. [Crossref] [PubMed]
- Oguro S, Esaki M, Kishi Y, et al. Optimal indications for additional resection of the invasive cancer-positive proximal bile duct margin in cases of advanced perihilar cholangiocarcinoma. Ann Surg Oncol 2015;22:1915-24. [Crossref] [PubMed]
- Hu HJ, Mao H, Tan YQ, et al. Clinical value of preoperative serum CA19-9 and CA 125 levels in predicting the resectability of hilar cholangiocarcinoma. Springerplus 2016;5:551. [Crossref] [PubMed]
- Wang JK, Hu HJ, Shrestha A, et al. Can preoperative and postoperative CA19-9 levels predict survival and early recurrence in patients with resectable hilar cholangiocarcinoma? Oncotarget 2017;8:45335-44. [PubMed]
- Endo I, House MG, Klimstra DS, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol 2008;15:2104-12. [Crossref] [PubMed]
- Shingu Y, Ebata T, Nishio H, et al. Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery 2010;147:49-56. [Crossref] [PubMed]
- Lee JH, Hwang DW, Lee SY, et al. The proximal margin of resected hilar cholangiocarcinoma: the effect of microscopic positive margin on long-term survival. Am Surg 2012;78:471-7. [Erratum appears in Am Surg. 2013 Jan;79(1):118]. [PubMed]
- Ma W-J, Shrestha A, Li F-Y. Is intraoperative frozen section analysis of the proximal bile ducts in hilar cholangiocarcinoma of limited value? Cancer Med 2016;5:2848-9. [Crossref] [PubMed]
- Mantel HTJ, Westerkamp AC, Sieders E, et al. Intraoperative frozen section analysis of the proximal bile ducts in hilar cholangiocarcinoma is of limited value. Cancer Med 2016;5:1373-80. [Crossref] [PubMed]
- Hu HJ, Mao H, Shrestha A, et al. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: A single-institution experience in China. World J Gastroenterol 2016;22:2601-10. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. Springer; 2017.
- Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 2003;238:73-83. [Crossref] [PubMed]
- Otto G, Hoppe-Lotichius M, Bittinger F, et al. Klatskin tumour: meticulous preoperative work-up and resection rate. Z Gastroenterol 2011;49:436-42. [Crossref] [PubMed]
- Schiffman SC, Reuter NP, McMasters KM, et al. Overall survival peri-hilar cholangiocarcinoma: R1 resection with curative intent compared to primary endoscopic therapy. J Surg Oncol 2012;105:91-6. [Crossref] [PubMed]
- Cannon RM, Brock G, Buell JF. Surgical resection for hilar cholangiocarcinoma: experience improves resectability. HPB 2012;14:142-9. [Crossref] [PubMed]
- Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the "new era": the Nagoya University experience. J Hepatobiliary Pancreat Sci 2010;17:449-54. [Crossref] [PubMed]
- Okazaki Y, Horimi T, Kotaka M, et al. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology 2002;49:625-7. [PubMed]
- Bosma A. Surgical pathology of cholangiocarcinoma of the liver hilus (Klatskin tumor). Semin Liver Dis 1990;10:85-90. [Crossref] [PubMed]
- Choi AR, Park JC, Kim JH, et al. High level of preoperative carbohydrate antigen 19-9 is a poor survival predictor in gastric cancer. World J Gastroenterol 2013;19:5302-8. [Crossref] [PubMed]
- Chen CC, Yang SH, Lin JK, et al. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res 2005;124:169-74. [Crossref] [PubMed]
- Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 Levels Can Predict Stage and Survival in Patients With Resectable Pancreatic Adenocarcinoma. J Clin Oncol 2006;24:2897-902. [Crossref] [PubMed]
- Singh S, Tang S-j, Sreenarasimhaiah J, et al. The Clinical Utility and Limitations of Serum Carbohydrate Antigen (CA19-9) as a Diagnostic Tool for Pancreatic Cancer and Cholangiocarcinoma. Dig Dis Sci 2011;56:2491-6. [Crossref] [PubMed]
- Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg 2009;198:333-9. [Crossref] [PubMed]
- Mann DV, Edwards R, Ho S, et al. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol 2000;26:474-9. [Crossref] [PubMed]
- Kim HJ, Kim MH, Myung SJ, et al. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol 1999;94:1941-6. [Crossref] [PubMed]
- Tempero MA, Uchida E, Takasaki H, et al. Relationship of Carbohydrate Antigen 19-9 and Lewis Antigens in Pancreatic Cancer. Cancer Res 1987;47:5501-3. [PubMed]
- Narimatsu H, Iwasaki H, Nakayama F, et al. Lewis and Secretor Gene Dosages Affect CA19-9 and DU-PAN-2 Serum Levels in Normal Individuals and Colorectal Cancer Patients. Cancer Res 1998;58:512-8. [PubMed]
- Ebata T, Watanabe H, Ajioka Y, et al. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg 2002;89:1260-7. [Crossref] [PubMed]
- Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma - A histologic analysis of 62 resected cases. Ann Surg 1998;227:405-11. [Crossref] [PubMed]
- Nakanuma Y, Miyata T, Uchida T. Latest advances in the pathological understanding of cholangiocarcinomas. Expert Rev Gastroenterol Hepatol 2016;10:113-27. [Crossref] [PubMed]
- Somer L, Andrejic B, Milosevic P. Origin and pathological characteristics of Klatskin tumor: a case report and literature review. Pol J Pathol 2012;63:65-70. [PubMed]
- Bhuiya MR, Nimura Y, Kamiya J, et al. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg 1992;215:344-9. [Crossref] [PubMed]
- Saxena A, Chua TC, Chu FC, et al. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg 2011;202:310-20. [Crossref] [PubMed]
- Kuang D, Wang GP. Hilar cholangiocarcinoma: Pathology and tumor biology. Front Med China 2010;4:371-7. [Crossref] [PubMed]