The first-line treatment for unresectable hepatocellular carcinoma patients: lenvatinib versus sorafenib, or beyond?
In the past decade, sorafenib has been the only approved molecular-targeted agent for unresectable hepatocellular carcinoma (uHCC) without any authentic challenges. There has been an urging need for alternatives or superior options for a long while (1-3). At least 25 molecular-targeted drugs emerged in the past 15 years and had been tested for the efficacy and safety in treating uHCC, yet most of those trials have failed to show positive results (Table 1).
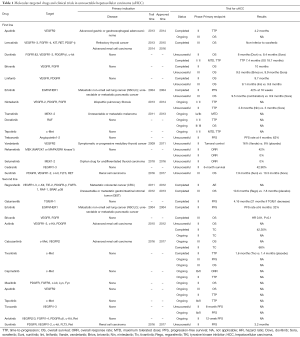
Full table
Recently, an open-label, randomized, global multi-center, non-inferiority trial (REFLECT) was completed and published with exciting results that may be the greatest breakthrough in the last decade. The trial compared the efficacy and safety of lenvatinib versus sorafenib as the first-line systemic treatment for patients with uHCC. The primary endpoint was non-inferiority in overall survival (OS) with a predefined non-inferiority margin of 1.08, which was based on the results of previous phase III trials of sorafenib. A total of 954 patients with uHCC, Barcelona Clinic Liver Cancer Group stage B or C, Child-Pugh class A, and no prior systemic therapy, were enrolled and randomized into two groups (lenvatinib: 478 patients; sorafenib: 476 patients). Starting dose of lenvatinib was 8 mg for patients <60 kg, or 12 mg for patients ≥60 kg once-daily, and sorafenib was administered at a dose of 400 mg twice-daily. The results showed no significant difference in OS between the two groups, non-inferiority in the primary OS endpoint was statistically confirmed (13.6 vs. 12.3 months; HR, 0.92; 95% CI, 0.79–1.06). The progression-free survival (7.4 vs. 3.7 months), time to progression (8.9 vs. 3.7 months) and overall response rate were statistically more favorable in the lenvatinib group comparing to the sorafenib group. The most common treatment-emergent adverse event among patients who received lenvatinib were hypertension, diarrhoea, dropped appetite, and decreased weight, while the most frequently occurred side effect in the sorafenib group were palmar-plantar erythrodysesthesia, diarrhoea, hypertension, and decreased appetite. The adverse events rate adjusted by patient-year was 18.9 episodes per patient-year in the lenvatinib group and 19.7 episodes per patient-year in the sorafenib group. The incidence of grade 3 or higher side effect was comparable, 3.2 and 3.3 patient-year respectively. Fatal adverse events occurred in 11 (2%) patients treated with lenvatinib and 4 (1%) patients in sorafenib group. The overall adverse events rate of lenvatinib group was consistent with previous phase II trial and was not significantly different from that of sorafenib group (4).
Despite of the fact that the study included experimental data from 154 research centers distributed in 20 countries across the Asia-Pacific, Europe, and North America, the actual population enrolled in the study was rather small. Among the 954 patients with uHCC that were included in the study, 67% were from China and Japan, while the proportion of patients from other area was insufficient. Seventy percent of the recruited patients were infected with hepatitis, the majority being Chinese and Japanese, with 20% of whom infected with hepatitis C and 50% suffered from hepatitis B. Though the researchers conducted detailed subgroup analyses, such as body weight, region, etc., the response of hepatocellular carcinoma to targeted drugs might vary according to the hepatitis virus they were burdened with. That this study did not consider the etiology as a stratification factor is a disadvantage (5). In addition, the phase III clinical trial of sorafenib in 2007 did not explicitly exclude patients with biliary invasion or tumor volume exceeding 50% of the total liver volume (6). However, this portion of the patients was excluded from this study, herein the safety and efficacy of lenvatinib in those patients were not demonstrated distinctively.
Since sorafenib had been approved for the first-line therapy for patients with uHCC in 2007, many other molecular-targeted agents were developed and tested in clinical trials for superior efficacy or safety in the first-line treatment of uHCC. To date, only lenvatinib has passed Phase III clinical trial. The first-line treatments sunitinib, brivanib and linifanib have all failed the phase III clinical trials. Combination of erlotinib and sorafenib, doxorubicin and sorafenib, Y90 and sorafenib, and sorafenib with hepatic arterial infusion chemotherapy were unsuccessful in phase III clinical trials either. Among the second-line drugs, regorafenib and cabozantinib are the only drugs that passed phase III clinical trials, and regorafenib has already been approval by the Food and Drug Administration (FDA) as a second-line treatment for uHCC patients. Targeted drug apatinib has reliable safety and efficacy for patients with uHCC. A phase II clinical trial confirmed that apatinib combined with TACE displayed an exciting application potential for liver cancer (7), and a phase III clinical trial of apatinib as a second-line treatment for uHCC patients will be completed by the end of June, the results of which are widely anticipated.
In addition, immune checkpoint inhibitors have shed a new light on HCC treatment. In 2017, the FDA accelerated the approval of an anti-programmed death-1 (PD-1) antibody, nivolumab, as a second-line medication for patients with uHCC who received sorafenib treatment, and a phase III superiority clinical trial for nivolumab as first-line treatment of uHCC is underway. A phase II non-controlled clinical trial showed that tremelimumab, an antibody against CTLA-4, could also benefit the patients with uHCC (8), and a phase III clinical trial of a combination of tremelimumab and durvalumab, launched officially in October 2017, is now recruiting.
Currently, the efficacy of targeted drugs, including sorafenib, on uHCC is modest, and lenvatinib is the first novel molecular-targeted agent to challenge sorafenib in the first-line treatment of uHCC for the past 10 years. With the emerging of innovative molecular-targeted agents and new therapeutic strategies (especially immunotherapy), the combination of different targeted agents or immunotherapy is also a trending topic in future research. Can those researches bring more benefit to liver cancer patients? We will find the answer in the near future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Forner A, Reig M, Bruix J.. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Coskun A, Yavasoglu I, Yasa MH, et al. Cetirizine-induced hepatotoxicity: case series and review of the literature. Gastroenterol Rep (Oxf) 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Li C, Li R, Zhang W. Progress in non-invasive detection of liver fibrosis. Cancer Biol Med. 2018;15:124-36. [Crossref]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Elwir S, Hal H, Veith J, et al. Radiographical findings in patients with liver cirrhosis and hepatic encephalopathy. Gastroenterol Rep (Oxf) 2016;4:221-5. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol Ther 2017;18:433-8. [Crossref] [PubMed]
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]


