Maintenance with single agent bevacizumab fails to improve disease-control in metastatic colorectal cancer
The availability of biologicals, such as anti-EFGR and anti-VEGF antibodies in combination with chemotherapy (ChT), has improved prognosis of metastatic colorectal cancer (mCRC). However, the administration of drug combinations for a prolonged time implies an increased rate of toxicities, which is why some strategies to de-escalate treatment intensity have been studied.
In the phase III PRODIGE 9 study, a strategy of bevacizumab maintenance was compared to no maintenance at all with chemotherapy-free intervals (CFI), after an induction therapy consistent in 12 cycles of FOLFIRI-bevacizumab (1) (Table 1). At progression, a sequence of 8 cycles of FOLFIRI-bevacizumab was reintroduced, followed by another CFI or maintenance with bevacizumab; this sequence was repeated until progressive disease (PD) on ChT. The primary endpoint was the tumor control duration (TCD), defined as the time elapsed between randomization, and tumor progression during a ChT sequence. In this study, the primary endpoint of median TCD was not significantly different, being 15 months in both groups. However, ChT was only reintroduced in 60.2% of patients in the maintenance arm and 69.5% in the observation arm. In the pre-planned per-protocol analysis (considering 261 patients with at least one reintroduction of ChT), median TCD was 17.8 months in the maintenance arm and 23.3 months in the observation arm, but the difference was not significant. Therefore, the study failed to demonstrate a superiority of maintenance bevacizumab over observation. Besides, no significant differences were observed in median progression free survival (PFS) or overall survival (OS) between the study arms. Other secondary endpoints such as assessment of quality of life were no different, but toxicities in the CFI were more frequent in the maintenance arm, particularly cardiovascular toxicities. The authors concluded that bevacizumab maintenance monotherapy did not improve TCD, CFI, PFS or OS.
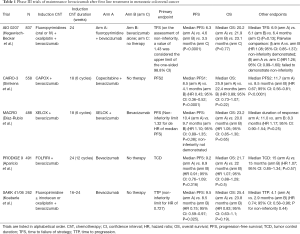
Full table
Other studies have explored the role of bevacizumab as maintenance treatment after an induction ChT. In the NO16966 study, bevacizumab or placebo added to first-line oxaliplatin-based ChT was continued until PD or for 48 weeks. The addition of Bevacizumab prolonged PFS but no differences in OS were observed. The duration of treatment was the same in the two groups, and the authors suggested that continuing bevacizumab until PD may be necessary to maximize its efficacy (2). In the phase III MACRO study, six cycles of induction XELOX-bevacizumab were followed by maintenance treatment with the same treatment or bevacizumab alone (3). This study failed to demonstrate the non-inferiority of maintenance with bevacizumab monotherapy, but due to the small difference in PFS or OS between arms, the authors suggested than single-agent bevacizumab could be a potential alternative to maintenance treatment. Afterwards, the SAKK 41/06 phase III non-inferiority trial compared also continuation of bevacizumab vs. no treatment after 4-6 months of first-line induction ChT (4). The primary endpoint of median time to progression (TTP) was not significantly different (4.1 months with bevacizumab vs. 2.9 months with observation), and non-inferiority could not be demonstrated. The short benefit on the disease control obtained with maintenance with bevacizumab as single agent, reinforce the need for alternative strategies.
Subsequently, in the phase III study CAIRO-3 maintenance treatment with capecitabine and bevacizumab was compared to observation (5). As in the PRODIGE 9 study, induction treatment was reintroduced at the first progression. The primary end point was PFS2 (the interval between randomization and the date of second progression) and it was significantly improved with maintenance treatment, at the expense of more grade 3 hand-food syndrome, but with preservation of quality of life. The median OS was not significantly different between the study arms, but it should be noted, however, that those patients who achieved a complete or partial response to induction treatment experienced a greater benefit from maintenance bevacizumab.
In the phase III AIO 0207 trial, after first-line induction oxaliplatin-based plus bevacizumab ChT, patients were randomly assigned to either maintenance with fluoropyrimidines and bevacizumab, bevacizumab alone or observation (6). The primary end point of time to failure of strategy (TFS), defined as the time from randomization to second progression after maintenance, death, or initiation of further treatment, showed no significant differences between the three arms. Bevacizumab alone demonstrated to be a non-inferior treatment compared to maintenance treatment with fluoropyrimidines plus bevacizumab, but when the second approach was compared with the observation arm the non-inferiority was not demonstrated. PFS was improved with maintenance therapy with fluoropyrimidines and bevacizumab, but no differences in OS were observed.
Nowadays there are no relevant biomarkers to take into account when deciding the best maintenance strategy after first line ChT on mCRC. A subgroup analysis in the AIO 0207 trial showed that patients with RAS or RAF mutation had longer time to first progression in the doublet treatment compared with the other two groups (6). In contrast, in the extended molecular subgroup analyses of the CAIRO3 study, RAS/BRAF wild type patients showed to have more benefit from the maintenance treatment than from observation for the primary endpoint, than RAS-mutant patients (7). In this analyses sidedness of tumor was also studied, and although right-sided tumors were seen to have inferior prognosis, both patients, right and left-sided, showed benefit from maintenance.
Recently, a meta-analysis evaluated a bevacizumab-based maintenance strategy in comparison with a CFI or continuation of therapy after induction ChT (8). Five trials were included in the quantitative analysis (MACRO, a study by Yalcin et al. CAIRO-3, SAKK and AIO 0207) (3-6,9). The primary analysis compared bevacizumab-based maintenance therapy with complete stop of therapy, showing a significant benefit for maintenance treatment on PFS and TFS, with a trend in favor of maintenance therapy for OS. The authors suggested that the unbalanced use of reintroduction treatment because of toxicity or at investigator discretion, reduced the effect of maintenance therapy in terms of TFS. In the secondary analysis comparing continuation of induction ChT vs. bevacizumab maintenance, no statistically significant differences in OS or TFS were seen.
According to the published studies evaluating the role of bevacizumab and fluoropyrimidines as maintenance strategies, we cannot recommend observation after induction ChT, as it has not demonstrated non-inferiority with respect to bevacizumab alone or bevacizumab-based maintenance treatment. Maintenance with a bevacizumab-fluoropyrimidine doublet after induction ChT should be the priority option, as it has proved to improve time to failure of treatment, PFS and maybe OS, as seen in the CAIRO-3 study. On the other hand, continuing bevacizumab alone after induction ChT could be an option for those patients who present toxicity to fluoropyrimidines, as studies suggest that bevacizumab is superior to observation alone and it may improve PFS. We still do not have information enough about which groups of patients could benefit from observation after induction ChT, as data published to date are conflicting with respect to age, sidedness, RAS mutation, tumor burden and other factors. It should be outlined that patients with BRAF mutant mCRC have a poor prognosis and they should not be enrolled in de-escalation strategies. Additional research is needed to identify biomarkers of response to maintenance bevacizumab-based treatment in order to define subgroups which could undergo observation and a real CFI.
Acknowledgements
Funding: This work was supported in part by grants from the Spanish Government, Ministerio de Economía y Competitividad (FIS PI12/02767 and PI15/02180 to AC) and Generalitat Valenciana (PROMETEO 2013-005 to AC) and CIBERONC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Aparicio T, Ghiringhelli F, Boige V, et al. Bevacizumab Maintenance Versus No Maintenance During Chemotherapy-Free Intervals in Metastatic Colorectal Cancer: A Randomized Phase III Trial (PRODIGE 9). J Clin Oncol 2018;36:674-81. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Díaz-Rubio E, Gómez-España A, Massutí B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 2012;17:15-25. [Crossref] [PubMed]
- Koeberle D, Betticher DC, von Moos R, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol 2015;26:709-14. [Crossref] [PubMed]
- Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015;385:1843-52. [Crossref] [PubMed]
- Hegewisch-Becker S, Graeven U, Lerchenmüller CA, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2015;16:1355-69. [Crossref] [PubMed]
- Goey KK, Elias SG, van Tinteren H, et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol 2017;28:2128-34. [Crossref] [PubMed]
- Tamburini E, Rudnas B, Santelmo C, et al. Maintenance based Bevacizumab versus complete stop or continuous therapy after induction therapy in first line treatment of stage IV colorectal cancer: A meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 2016.115-23. [Crossref] [PubMed]
- Yalcin S, Uslu R, Dane F, et al. Bevacizumab + capecitabine as maintenance therapy after initial bevacizumab + XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III ‘Stop and Go’ study results--a Turkish Oncology Group Trial. Oncology 2013;85:328-35. [Crossref] [PubMed]


