
When lifestyles sign
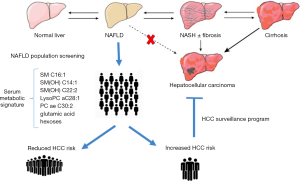
Nonalcoholic fatty liver disease (NAFLD) has a global prevalence of 25%, reflecting the global epidemic of obesity and diabetes (1). The onset and development of NAFLD are closely associated with dietary habits and lifestyle; consequently, lifestyle modifications including weight loss, increased physical activity, and dietary changes remain the treatment of choice for NAFLD. Even when drugs to treat non-alcoholic steatohepatitis will be available, lifestyle changes will remain an essential component of the therapeutic plan of these patients. In the last years, a strong association between lifestyle and hepatocellular carcinoma (HCC) has become evident (2-4). In patients with NAFLD, the annual incidence of HCC is 0.44 per 1,000 person/year and almost 50% of cases develops in a non-cirrhotic liver resulting in a diagnosis at an advanced stage with a worse prognosis (1,5). Nonetheless, the high prevalence of NAFLD with the relatively low risk for HCC makes a surveillance program cost-ineffective in non-cirrhotic patients (6). In this scenario, the need to identify NAFLD patients at higher risk of developing HCC is a priority. In this direction, Assi et al. published recently 2 interesting papers showing a serum metabolic signature reflecting a healthy lifestyle and associated with a reduction in HCC risk (7,8). They identified 147 cases of HCC and 147 matched controls in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, a multinational prospective European cohort in which questionnaire and interview data about diet habits, lifestyle, medical history and anthropometric measures have been collected at baseline and at regular intervals during the enrollment period from apparently healthy participants, who were followed over time for the occurrence of cancer and other diseases (9). Within all these collected data, Assi and colleagues focus their attention on 7 lifestyle variables of the EPIC-modified healthy lifestyle index (HLI): body mass index (BMI), lifetime alcohol intake, a diet score, physical activity, smoking, diabetes and hepatitis infection. Then on serum samples of the 294 identified cases and controls, they measured concentrations of a set of known metabolites using targeted metabolite profiling approach, resulting in a total of 132 metabolites included in the 2 studies, after the exclusion of the one with >40% of missing values. With two different methodological approaches they evaluated the relationship between lifestyle factors, metabolic profile and HCC risk within the same nested case-control study design. In their work published on the American Journal of Clinical Nutrition the authors performed a Partial Least Square (PLS) analysis relating a modified HLI to targeted serum metabolomics and liver function, to assess the HCC risk (7), while in the paper published on Cancer Epidemiology, Biomarkers and Prevention, they used the “meeting-in-the-middle” (MITM) principle through a mediation analysis to identify a biomarker mediator of the relationship between individual lifestyle factors and HCC risk (8). Using the PLS analysis, they showed an association between the 7 mentioned lifestyle variables and 7 metabolites. In particular the modified HLI was associated positively with 3 sphingomyelins [SM C16:1, SM(OH) C14:1, and SM(OH) C22:2], and 2 phosphatidylcholines [lysophosphatidylcholine (LysoPC) aC28:1 and acyl-alkyl phosphatidylcholine (PC ae) C30:2], and negatively with glutamic acid and hexoses (7). Then they evaluated separately in conditional regression models the lifestyle and metabolic signatures and the HCC risk, showing that this “healthy lifestyle metabolic signature” is associated with a reduction of 72% (95% CI: 57–82%) in HCC risk. In particular, this metabolic signature maintains its inverse association with HCC risk after excluding the hepatitis-positive cases (OR =0.33), so in the setting of alcoholic and non-alcoholic liver diseases (7). In a second study they performed a mediation analysis assessing to investigate whether the metabolic signature mediated the relation between the previous mentioned lifestyle factors and the risk of HCC (8), showing that the association between each lifestyle factors with HCC risk is strongly mediated by the signature, with the exception of physical activity and hepatitis infection [mediator effect: 0.90 (0.60–1.35) and 1.22 (0.88–1.69), respectively] (8). However, a correlation is not providing any information about the critical level of a metabolite that should be used for discerning higher and lower risk of HCC. The authors are not giving any cut-off value in the metabolite concentration to discern low risk to high risk patients. In addition, it seems that the AUCs for these metabolites are not high enough to be used as a sensitive and specific metabolic signature. Moreover, the authors did not correlate this “metabolic signature” with a possible regulation mechanism of biological pathways. They used a targeted approach; a non-targeted profiling might be beneficial for understanding this into more details. Finally, to be translated to a widely available blood-based metabolic tool, it is mandatory to validate this signature in an independent and prospective cohort, since a limitation of both studies is the rather small sample size and the retrospective design. In any case, this metabolic signature linked to healthy lifestyle sounds like a promising tool to predict disease progression and HCC onset identifying “at-risk patients” in the setting of non-cirrhotic NAFLD patients in a context where “-omics” signatures have already been explored (10-12). In our conceptual diagram (Figure 1), we lay out how the availability of a population screening based on a validated serum metabolic signature can maximize the efforts of the hepatology community in the identification of a subset of patients with an increased risk of HCC, for whom the enrollment in dedicated HCC surveillance program is mandatory. In addition, this metabolic signature could be used as starting point to address current gaps in our NAFLD understanding of progression and carcinogenesis.
Acknowledgements
None.
Footnote
Conflicts of Interest: JF Dufour has the following conflicts of interest—advisory committees: Abbvie, Bayer, BMS, Falk, Genfit, Genkyotex, Gilead Science, Intercept, Lilly, Novartis; speaking and teaching: Abbvie, Bayer, BMS, Genfit, Gilead Science, Novartis. The other authors have no conflicts of interest to declare.
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. [Crossref] [PubMed]
- Saran U, Humar B, Kolly P, et al. Hepatocellular carcinoma and lifestyles. J Hepatol 2016;64:203-14. [Crossref] [PubMed]
- Saran U, Guarino M, Rodríguez S, et al. Anti-tumoral effects of exercise on hepatocellular carcinoma growth. Hepatol Commun 2018;2:607-20. [Crossref] [PubMed]
- Piguet AC, Saran U, Simillion C, et al. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol 2015;62:1296-303. [Crossref] [PubMed]
- Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016;63:827-38. [Crossref] [PubMed]
- European Association for the Study of the Liver, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Assi N, Gunter MJ, Thomas DC, et al. Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort. Am J Clin Nutr 2018;108:117-126. [PubMed]
- Assi N, Thomas DC, Leitzmann M, et al. Are Metabolic Signatures Mediating the Relationship between Lifestyle Factors and Hepatocellular Carcinoma Risk? Results from a Nested Case-Control Study in EPIC. Cancer Epidemiol Biomarkers Prev 2018;27:531-40. [Crossref] [PubMed]
- Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113-24. [Crossref] [PubMed]
- Alonso C, Fernández-Ramos D, Varela-Rey M, et al. Metabolomic Identification of Subtypes of Nonalcoholic Steatohepatitis. Gastroenterology 2017;152:1449-61.e7. [Crossref] [PubMed]
- Barr J, Caballería J, Martínez-Arranz I, et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res 2012;11:2521-32. [Crossref] [PubMed]
- Cazanave S, Podtelezhnikov A, Jensen K, et al. The Transcriptomic Signature Of Disease Development And Progression Of Nonalcoholic Fatty Liver Disease. Sci Rep 2017;7:17193. [Crossref] [PubMed]


