
Cabozantinib for advanced hepatocellular carcinoma
Introduction
Results of the phase III CELESTIAL trial of cabozantinib were recently reported in the New England Journal of Medicine (1). Whereas all preceding clinical trials examining second-line agents ended in failure (2-7), the CELESTIAL trial succeeded, and cabozantinib has become the fourth molecular-targeted agent for hepatocellular carcinoma (HCC). This success was followed by another clinical trial of ramucirumab, the success of which was reported at the American Society of Clinical Oncology meeting in June 2018. As a result, two first-line agents, sorafenib and lenvatinib (8), and three second-line agents, regorafenib, cabozantinib, and ramucirumab, are now available for the treatment of HCC.
Characteristics of cabozantinib
The chemical structure of cabozantinib is relatively similar to that of regorafenib (9,10). However, the kinase inhibitory activity (IC50) of cabozantinib is quite different from that of regorafenib. Although cabozantinib is generally known as a dual inhibitor of VEGFR-2 and c-MET (11,12), compared with regorafenib it is a more potent inhibitor of MET, AXL, and TIE-2. VEGF, MET, and AXL are deeply involved in tumor growth and angiogenesis. MET and AXL are involved in acquisition of resistance to anti-angiogenic agents (11,13). Also, expression of VEGF, MET, and AXL is a known predictor of poor prognosis (14,15).
A waterfall plot from the phase II trial showed tumor reduction in a considerable proportion of patients. Progression-free survival (PFS) was 4.2 months in sorafenib-naïve patients and 5.5 months in sorafenib-treated patients; overall survival (OS) was 11.5 months. Given that some participants had received first-line therapy, the overall response rate (ORR) of 5%, the disease control rate (DCR) of 81%, and PFS of 5.2 months were not particularly good compared to the results of the phase II trial of regorafenib (16). Also, adverse event (AE) profiles showed that AEs were slightly more common with cabozantinib than with regorafenib (12).
Phase III CELESTIAL trial
In light of these results, cabozantinib proceeded to a phase III CELESTIAL trial. The study design was not as sophisticated or well thought out as the RESORCE trial’s (17). For example, use of “vascular invasion and/or extrahepatic spread” as a stratification factor posed a potential risk of a disadvantageous imbalance in vascular invasion. In fact, such a disadvantageous imbalance occurred in the BRISK-PS trial that ended in failure. Further, alpha-fetoprotein (AFP) was not included among the stratification factors, posing a potential risk of a disadvantageous imbalance as actually seen in the REFLECT trial. After the RESORCE trial, use of vascular invasion as an independent stratification factor, along with the use of AFP as a stratification factor, became a standard trial design for second-line agents (18). However, the design of this phase III trial was conventional and lacked the sophistication seen in some other trials. For example, exclusion of sorafenib-intolerant patients, a criterion used in the RESORCE trial, was not applied in this phase III trial. The inclusion criteria related to prior treatment in this trial were (I) prior sorafenib treatment; (II) disease progression following at least one prior systemic treatment for HCC; and (III) up to two prior systemic regimens for advanced HCC. The proportion of sorafenib-intolerant participants was not reported.
A total of 707 patients with progression of unresectable HCC following at least 1 prior systemic treatment with sorafenib between September 2013 and September 2017 were enrolled in this trial, and the second interim analysis in January 2016 demonstrated superiority in the primary endpoint OS. This successful clinical trial showed significantly longer OS in the cabozantinib group (10.2 months; 95% CI, 9.1–12.0 months) than in the placebo group (8.0 months; 95% CI, 6.8–9.4 months). PFS, a secondary endpoint, was also longer in the cabozantinib group (5.2 months; 95% CI, 4.0–5.5) than in the placebo group (1.9 months; 95% CI, 1.9–1.9). Because neither vascular invasion nor extrahepatic spread (EHS) was used independently for stratification, imbalances in patient characteristics were observed between the two groups. Specifically, there was a favorable imbalance in the proportion of patients with macrovascular invasion (MVI): 27% in the cabozantinib group versus 34% in the placebo group. MVI is a well-known extremely strong predictor of poor prognosis. OS values in patients with and without MVI were 5.3 and 9.7 months, respectively, in the placebo group, and 7.6 months and 12.4 months, respectively, in the cabozantinib group, suggesting that the above imbalance had some influence on the trial outcomes. Also, HBV was the major etiology of HCC in this trial (38% of participants with HBV versus 24% with HCV), and the hazard ratio (HR) for OS was 0.69 in those with HBV but 1.11 in those with HCV. The HR for PFS was 0.31 in patients with HBV while 0.61 in those with HCV, indicating that cabozantinib may be more effective in those with HBV.
PFS of 1.9 months in the placebo group was quite short, which is the second shortest after the PFS of 1.5 months in the RESORCE trial among previous clinical trials for second-line agents (Figure 1). This means that the CELESTIAL trial, like the trial for regorafenib, might have included a small number of sorafenib-intolerant patients, and in those patients, the disease progressed during the sorafenib-treated period, and then progressed further and rapidly during the placebo-treated period. The median length of prior sorafenib treatment was relatively long (5.3 months) in the CELESTIAL trial, suggesting that many patients were with stable disease (SD) for long time. Incidentally, the median duration of prior sorafenib treatment was 7.8 months in the trial for regorafenib. This suggests the possibility that patients who were more responsive to sorafenib were included in the trial, which in turn resulted in the favorable outcome for patients treated with the testing agent. Also, the percentages of patients who received post-trial treatment was comparable in the cabozantinib group (25%) and in the placebo group (30%), suggesting that conditions were pretty poor in these groups. Thus, although not reported, the proportion of sorafenib-intolerant patients might have been relatively small in this trial, which resulted in the favorable outcome.
Comparison between regorafenib and cabozantinib: efficacy and safety
Comparison of OS, ORR, and FPS indicates that efficacy is roughly similar between cabozantinib and regorafenib. Even in the subgroup of patients who received sorafenib alone during prior treatment, HRs for PFS and OS were comparable between the CELESTIAL trial and the RESORCE trial: 0.40 vs. 0.46 for PFS, and 0.70 vs. 0.63 for OS (Table 1). The CELESTIAL trial showed OS of 8 months in placebo-treated patients, which was roughly same compared to the previous phase III trials for second-line agents, and OS of 10.2 months in the cabozantinib-treated patients, which was similar to the OS in regorafenib-treated patients in the RESORCE trial, the only other positive trial. Compared with these positive trials, three previous unsuccessful trials (BRISK-PS, EVOLVE, and REACH trials) showed shorter OS in patients treated with second-line agents despite similar OS in the placebo group, indicating that the efficacy of cabozantinib is as good as that of regorafenib. Similarly, PFS in the placebo-treated patients was very short, but that in cabozantinib-treated patients was longest (5.2 months), clearly indicating its favorable efficacy (Figure 1).
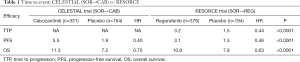
Full table
The treatment durations were comparable between cabozantinib (3.8 months) and regorafenib (3.6 months), indicating acceptable tolerability of these agents. Dose reduction and treatment discontinuation due to AEs occurred more frequently with cabozantinib than with regorafenib. Palmar-plantar erythrodysesthesia, diarrhea and asthenia were more common with cabozantinib than with regorafenib, indicating that the toxicity may be slightly higher for cabozantinib than for regorafenib. However, given strict exclusion of sorafenib-intolerant patients in the RESORCE trial, cabozantinib and regorafenib may be comparable in terms of AEs.
Key factors contributing to success of CELESTIAL trial
What were the key factors that contributed to the success of the CELESTIAL trial, despite toxicity possibly being slightly higher for cabozantinib and the lack of design sophistication (e.g., different from the RESORCE trial). There were six main factors:
- The antitumor effect of cabozantinib was sufficiently potent;
- Its toxicity and tolerability were acceptable;
- There was a favorable imbalance of vascular invasion for cabozantinib;
- Cabozantinib is effective in HBV patients, and the HBV patients were the largest subpopulation (38% of total) in the trial;
- Based on short time to progression and a low proportion of patients who received post-trial treatment, it is possible that a low proportion of sorafenib-intolerant patients were enrolled, thus could not readily received post-trial treatment because of poor general condition;
- Largest sample size [707] among the previous trials for second-line agents provided adequate power to detect small differences as significant.
Conclusions
The success of the clinical trial for cabozantinib expands the agents available for HCC treatment. Further, it will offer more treatment options, such as sequential therapy involving other molecular targeted agents, and advanced therapy in combination with immune checkpoint inhibitors, thereby considerably contributing to a better prognosis of HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ghassan K, Abou-Alfa GK, Meyer T, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol 2018;36:abstr 207.
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Kudo M, Moriguchi M, Numata K, et al. S-1 versus placebo in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE): a randomised, double-blind, multicentre, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:407-17. [Crossref] [PubMed]
- Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer 2017;6:101-12. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Pinna AD, et al. Efficacy and Safety of Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis of Phase III Trials. Liver Cancer 2017;6:337-48. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018. [Crossref] [PubMed]
- Lacy S, Hsu B, Miles D, et al. Metabolism and Disposition of Cabozantinib in Healthy Male Volunteers and Pharmacologic Characterization of Its Major Metabolites. Drug Metab Dispos 2015;43:1190-207. [Crossref] [PubMed]
- Strumberg D, Schultheis B. Regorafenib for cancer. Expert Opin Investig Drugs 2012;21:879-89. [Crossref] [PubMed]
- Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298-308. [Crossref] [PubMed]
- Kelley RK, Verslype C, Cohn AL, et al. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol 2017;28:528-34. [Crossref] [PubMed]
- Gay CM, Balaji K, Byers LA. Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer 2017;116:415-23. [Crossref] [PubMed]
- Zhu AX, Duda DG, Sahani DV, et al. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011;8:292-301. [Crossref] [PubMed]
- Ueki T, Fujimoto J, Suzuki T, et al. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology 1997;25:619-23. [Crossref] [PubMed]
- Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer 2013;49:3412-9. [Crossref] [PubMed]
- Kudo M. Regorafenib as Second-Line Systemic Therapy May Change the Treatment Strategy and Management Paradigm for Hepatocellular Carcinoma. Liver Cancer 2016;5:235-44. [Crossref] [PubMed]
- Kudo M. A New Era of Systemic Therapy for Hepatocellular Carcinoma with Regorafenib and Lenvatinib. Liver Cancer 2017;6:177-84. [Crossref] [PubMed]



