
What happened in 133 consecutive hepatic artery reconstruction in liver transplantation in 1 year?
Introduction
One of the most important steps during liver transplantation (LT) is the hepatic artery reconstruction (HAR). The surgical reconstruction technique is the prime risk factor for hepatic artery thrombosis (HAT). The use of microsurgical technique has helped to overcome the risk of HAT in many series (1-4). The higher magnification (10–15×) of the microscope helps in surveying accurately and identifying any intimal flaps or injury to the hepatic artery before the reconstruction. When the size of the vessel is less than 2 mm, reconstruction using loupes or a smaller magnification (<6×) can lead to problems and is risky. In addition, there may be a need to use alternate vessel for reconstruction when the recipient hepatic artery may not be available for reconstruction. Hence, to highlight the technical problems that were encountered and how they were tackled, we decided to look at our experience in the microvascular reconstruction of the hepatic artery in patients undergoing LT, over a period of 1 year, which gives a cross-sectional view of our routine practice in LT program at our center.
Methods
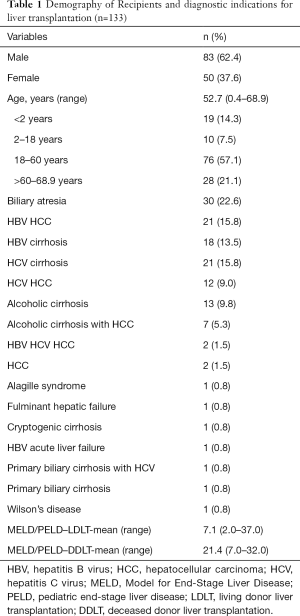
From January 2015 to December 2015, a total of 133 LTs were performed in Kaohsiung Chang Gung Memorial Hospital, Taiwan. There were 113 living donor liver transplantation (LDLT) and 20 deceased donor liver transplantation (DDLT). Seventeen livers from the deceased donors were harvested at CGMH, Kaohsiung itself. The other 3 livers were harvested from the other hospitals. Twenty-nine cases were pediatric and 104 cases were adults. The male to female ratio was 83/50. The median age of the group was 52.7 years (range, 0.4–68.9 years). The indication for LT were biliary atresia (22.6%), hepatitis B virus (HBV) hepatocellular carcinoma (HCC) (15.8%), HBV cirrhosis (13.5%), hepatitis C virus (HCV) cirrhosis (15.8%), HCV HCC (9.0%), alcoholic cirrhosis (9.8%), alcoholic cirrhosis with HCC (5.3%) and other causes (8.3%). The median Model for End-Stage Liver Disease (MELD) score was 17 in patients undergoing LDLT and was 21 in those undergoing DDLT (Table 1).
Full table
Surgical technique
All the hepatic artery reconstructions were completed using the posterior wall first, and a combined method for the anterior wall (5). The hepatic artery reconstructions were initiated following the completion of hepatic and portal vein reconstruction, once the graft was reperfused. All hepatic artery reconstructions were performed by a microvascular surgeon under an operating microscope with 6–15× magnification.
The technique of reconstructing the arteries was described fully in our previous report (5). Briefly, the microvascular reconstructions were performed using an 8-0 prolene or a 9-0 non-absorbable nylon monofilament suture (Ethilon, Ethicon Inc., Somerville, NJ, USA) on a 9-0 gauge Micropoint needle. The vessels’ posterior walls were reconstructed first using an interrupted suturing technique whereas the anterior wall was reconstructed using a continuous suturing and interrupted tying technique or a combined method. After that, the blood flow from the hepatic artery was immediately evaluated by a radiologist using a color Doppler ultrasound.
Postoperative care and follow-up
Cyclosporine-based immunosuppression was used for pediatric recipients, whereas, tacrolimus-based immunosuppression was employed for adults. Anticoagulation was administered immediately after the surgery. Similarly, after the transplantation, a Doppler ultrasound examination was performed to evaluate the blood flow in all the vessels daily during the first 2 weeks, every other day on the third week, and twice a week after that until discharge. A computed tomographic angiography (CTA) is performed to confirm any obstruction in the hepatic arteries if the Doppler examinations showed a poor or absence of flow in these vessels. The patient was immediately scheduled for surgery once an occlusion of hepatic artery was confirmed following the CTA, if done within 2 weeks after transplantation.
Statistics
Statistical analysis was performed using SPSS version 18.0 for windows. All the categorical data related to the patient’s baseline characteristics were presented as frequencies and percentages. All normally distributed continuous data was presented as mean with standard deviation and range.
Results
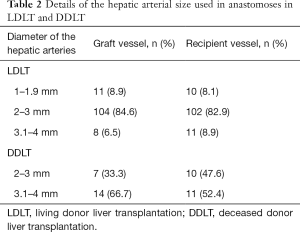
In the 113 patients who underwent LDLT, right lobe of the liver was used in 63 (55.8%) patients, whereas left lobe was used in 39 (34.5%) and left lateral lobe was used in 11 (9.7%) patients. One artery was anastomosed in 104 (92.0%) patients, two in 8 (7.1%) patients and three in 1 (0.9%) of the patient. From these liver grafts, right hepatic artery was used for anastomosis in 63 (55.8%) patients, left hepatic artery was used for anastomosis in 41 (36.3%) patients, left hepatic artery with segment 4 artery was used in 8 (7.1%) patients and left hepatic artery, right gastro-epiploic artery (RGEA) and cystic artery were used in 1 (0.9%) patient. Most of these arteries (84.6%) were 2–3 mm in size, 7 (6.5%) were between 3.1–4 mm in size and 11 (8.9%) were less than 2 mm in size (1–1.9 mm) (Tables 2,3).
Full table

Full table
The anastomosis patterns of the vessels between the liver graft and the recipient vessel, including the two or three vessel anastomoses are shown in Table 3. The commonest pattern was the right hepatic artery of the graft was anastomosed to either the left hepatic artery of the recipient in 27 (23.9%) patients or the right hepatic artery of the recipient in 24 (21.2%) patients. The second common pattern was the left hepatic artery was anastomosed to either the recipient’s right hepatic artery (16.8%) or the left hepatic artery (10.6%). Two cases (1.8%) needed radial artery graft (RAG) interposition graft and three cases (2.7%) needed reconstruction with RGEA. In the recipient, 8.1% (6) arteries used were smaller than 2 mm (1–1.9 mm). Majority (82.9%) were 2–3 mm in size (Table 2). The color Doppler evaluation done by a radiologist, immediately after the anastomosis, showed a mean Vmax 47±15 cm/s and a mean resistance index (RI) 0.65±0.14. The average time taken for anastomoses was 20–25 minutes.
In the 20 patients who underwent DDLT, whole liver was used as a graft in 9 (45.0%) patients, split right lobe in 5 (25.0%) patients and split left lobe was used in another 5 (25.0%) patients. Reduced liver graft was used in 1 (5.0%) patient. One artery was used for anastomosis in 19 (95.0%) patients and, two arteries were used in 1 (5.0%) patient. From these liver grafts, the proper hepatic artery was the most common used for anastomosis in 6 (30.0%) patients, followed by the common hepatic artery in 5 (25.0%) and LHA in 4 (20.0%) patients. Both the common hepatic artery and the splenic artery were used in 1 (5.0%) patients. The pattern of the arterial anastomoses is shown in Table 3. Most of the donor arteries were more than 3 mm (66.7%) in size. Most of the recipient arteries were also more than 3 mm (52.4%) (Table 2). The RGEA had to be used in one patient (5.0%). The colour Doppler evaluation done by a radiologist, immediately after the anastomosis, showed a mean Vmax 57.3±29 cm/s, mean RI 0.59±0.09. The average time taken for anastomoses was 15–20 minutes.
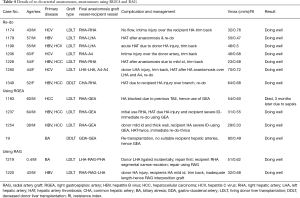
There were intimal dissections (IDs) involving either the donor (7) or the recipient (8) arteries of mild to severe nature in 9 (6.8%) patients. In 12 of them (9%), there was injury to the artery without ID. Immediately following graft arterial anastomosis, either there was poor flow (Vmax <0.20 m/s) or an intra-operative HAT was found in 9 (7.1%—8 LDLT, 4.8%—1 DDLT) patients. Immediate re-do anastomosis was done in all of these patients (Table 4).
Full table
Three patients (2.7%) of the LDLT group developed bile leak in the immediate postoperative period needing re-exploration and repair. Two (1.5%) patients needed an endoscopic retrograde biliary drainage (ERBD) and stenting for the biliary stricture. All these patients were followed up after surgery. At the time of writing this manuscript, the minimum duration of follow-up was 28 months and the maximum duration of follow-up was up to 36 months. There were three deaths (2.3%). One patient who underwent DDLT died immediately following the surgery because of diffuse intracerebral bleeding. One of the patients had an equivocal finding of HAT 19 days after LDLT, but was showing normal liver functions, hence was treated conservatively. Visualization of hepatic artery was noted 2 months after LDLT. He later developed hepatic abscess which was drained but ultimately died of sepsis at the end of 3 months. Since he had equivocal finding of the hepatic artery during follow-up and since the radiologist felt that visualized hepatic artery could be due collaterals, this could be a case of post-operative HAT treated conservatively. The third patient (LDLT group) developed in intrahepatic haematoma and herpes zoster. He also had an episode of gastrointestinal bleeding and finally succumbed to sepsis at the end of 10 weeks.
Discussion
This study was mainly taken up to look at the challenges during microvascular reconstruction of the hepatic artery in LT and also to see how we could overcome the same technically. It has been shown by various other studies in the literature that HAT following LT can be reduced from 25% without a microscope to less than 4% with a microscope (7-9). However, the microvascular surgeons may have to face some problems during the reconstruction. As it can be seen in Figure 1, we had problems like small arteries, need to do multiple reconstructions, hepatic arterial injury, ID, immediate HAT, need of re-doing the anastomosis, need to use RGEA and interposition RAG grafts.
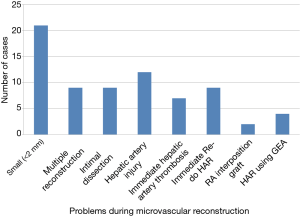
Liver grafts vessel or recipient vessel less than 2 mm diameter is considered to be a risk factor for HAT (10). Narrow diameter of the hepatic arteries complicates the reconstructive procedures both in adult and pediatric LDLTs (10). In our study, we had 21 (18.6%) instances, in the LDLT group, of either the donor vessels or the recipient vessels were less than 2 mm in size. In the past, liver graft with a vessel less than 2 mm was considered as a contraindication for transplantation (2,6,7). However, there was no statistical significance as comparison of result between the group with diameter less than 2 mm and the group with the diameter more than 2 mm. There was vessel size discrepancy in some of these cases which was overcome by cutting the smaller vessel obliquely and resorting to end to side anastomosis in one of the instances where a three vessel anastomosis was necessary. We also reconstructed all of the arteries with the pattern of obliquely cutting to prevent kinking or twist. Other methods like fish-moth method or funnelization method were not used in the present study.
In our previous study, we had noted that the incidence of multiple graft arteries even in right lobe graft could be 4.7% and was much higher in left lobe grafts (11). However, selective reconstruction of a single hepatic artery is sufficient, even in the presence of multiple arteries (12-14). Multiple reconstructions are usually associated with increase in the operation time, the duration of hepatic arterial reconstruction and blood loss. All these factors may increase the occurrence of a biliary stricture (15,16). Hence, many of the transplant surgeons avoid using a graft with multiple small vessels. But, still, we had to do multiple reconstructions in 9 (6.8%) cases, because both arteries were all significant in terms of angiosome in the graft.
The cause of hepatic arterial injury or ID could be because of excessive pulling during the dissection which may cause severe vasospasm and may also cause shedding of the intima. Another major cause is transarterial embolization (TAE) or chemoembolization to treat HCC patients. These procedures or the agents used in these procedures can damage the intima. The ID could be higher in patients undergoing TAE for HCC and has been classified as mild, moderate and severe (5). In mild and moderate ID, the HA can be used after trimming back but if ID is severe an alternative vessel needs to be used. Injury or ID can predispose to HAT. We had 9 (6.8%) cases of ID and 13 (9.8%) cases of hepatic artery injury in this study which had to be managed.
One of the unique features of this study was noticing the HAT intraoperatively, immediately after the anastomosis using the color Doppler and correcting the same immediately by re-doing the anastomosis. There were 7 (5.3%) such instances including the one in the DDLT. This immediate HAT was either due to vessel wall injury or the hepatic arterial ID. There were no instances of late (post-transplantation) re-do anastomosis in this study. As a result, all these patients had a good postoperative outcome. A late (>24 hours) re-do anastomosis could be associated with less desirable outcome (17). In this study we had to do immediate re-do anastomosis not only for HAT but also when we found poor flow (Vmax <0.20 m/s) or intimal injury following the anastomosis which would have led to HAT. The total number of re-do was in 9 (6.8%) cases. We also performed Doppler examination in all the vessels daily during the first 2 weeks, every other day on the third week, and twice a week after that until discharge. These could detect compromised circulation and re-do reconstruction earlier to prevent re-transplantation.
Gastric vessels can be used for hepatic arterial revascularization with good results (18). RGEA and left gastric artery (LGA) can be used in patients with ID of the recipient artery or those who develop HAT during thrombectomy and revascularization (18). The gastroepiploic artery has been used in coronary artery bypass graft surgery by the cardiac surgeons (19). The splenic artery has also been used in hepatic arterial revascularization but with the slight disadvantage of splenic infarction (20). In this study, we had to use RGEA in 4 (3%) cases. In two of the cases it was used as a re-do procedure to overcome HAT. In the other two cases it was used primarily as a substitute since recipient hepatic artery was blocked in one case due to previous TAE for HCC and in the other case the hepatic artery was not available due re-transplantation in a case of biliary atresia.
RGEA is our first choice as an alternative conduit for hepatic arterial reconstruction. However, if ID involves common hepatic artery or celiac axis, they are not suitable for the use. If RGEA is not available we choose the other alternative like RAG as interposition graft. When RGEA and LGA are anomalous or extremely short, RAG can be used as it offers a considerable length, appropriate diameter and excellent long-term patency (21). Ileocolic artery along with 17-cm RAG has been used as a secondary conduit for HA alternative in an adult LDLT (22). We had to use RAG in two cases (1.8%) of LDLT. In the first case, recipient vessel showed a narrow segment which had to be excised which resulted in shortening and hence RAG had to be used. In the second case, the recipient vessel showed ID and had to be trimmed which resulted in inadequate length and hence RAG had to be used. Radial artery is more similar as hepatic artery in term of diameter and resistance to compression. The venous graft is more vulnerable to be compressed by tissue. The radial artery was the first choice if interposition graft was needed.
The incidence of post-operative HAT in our study was 0.8% (one case of LDLT) which is one of the lowest. This patient had a doubtful diagnosis of HAT 19 days post LDLT and hence was managed conservatively and eventually had good flow from collaterals 2 months later.
There are arguments favoring arterial reconstruction using either a microscope or a loupe but not showing a statistical significance with respect to HAT (6,23-27); but there are no randomized trials. Our view is that loupes can give only a fixed working distance, fixed magnification, smaller field of view, limited Illumination, can cause weight burden on head and nose and can result in a spinal compression in a surgeon. On the other hand, microscope can give a changeable working distance, changeable magnification, large field of view, motorized focus and zoom, xenon Illumination, image/video recording and ergonomic device adjustment which are all advantageous if the technique is mastered. The higher magnification of the microscope helps in surveying accurately and identifying any intimal flaps or injury to the hepatic artery accurately before the reconstruction itself. When the size of the vessel is less than 2 mm, reconstruction using loupes or a smaller magnification (<6×) can lead to problems and is risky. In contrast, there was no contraindication using vessels less than 2 mm under microscope. We strongly recommend the use of microscope for all the hepatic artery reconstruction.
However, the causes for HAT can range from a simple technical factor to more complicated donor and recipient size, graft volume, graft vascular resistance, excessive portal pressure or flow etc. And now recently hypercoagulable states identified by thromboelastography have been added to the list of parameters (28,29). This study which gives a cross-sectional view represents probably the technical peak of the microvascular surgeon when the HAT could be at its lowest. Similar reports with no HAT have been reported (30,31).
To conclude, even with the technical advances in microsurgical reconstruction of hepatic artery, still small vessels or hepatic artery injury are the frequently encountered problems by a micro vascular surgeon. The other problems could be ID, need to do multiple reconstructions, immediate HAT and ability to re-do the same immediately. The micro vascular surgeon should have the capacity to use alternate conduits like RGEA or interposition grafts like RAG. With experience it is possible to bring down the HAT to the lowest possible level.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Chang Gung Medical Foundation on 2017/10/16 (No. 201701529B0).
References
- Inomoto T, Nishizawa F, Sasaki H, et al. Experiences of 120 microsurgical reconstructions of hepatic artery in living related liver transplantation. Surgery 1996;119:20-6. [Crossref] [PubMed]
- Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation—its surgical advantages compared with conventional procedures. Transplantation 1992;54:263-8. [Crossref] [PubMed]
- Hatano E, Terajima H, Yabe S, et al. Hepatic artery thrombosis in living related liver transplantation. Transplantation 1997;64:1443. [Crossref] [PubMed]
- Uchiyama H, Hashimoto K, Hiroshige S, et al. Hepatic artery reconstruction in living donor liver transplantation: review of its techniques and complications. Surgery 2002;131:S200-4. [Crossref] [PubMed]
- Lin TS, Chiang YC, Chen CL, et al. Intimal dissection of the hepatic artery following transarterial embolization for hepatocellular carcinoma: an intraoperative problem in adult living donor liver transplantation. Liver Transpl 2009;15:1553-6. [Crossref] [PubMed]
- Ikegami T, Nishizaki T, Uchiyama H, et al. Doubly-armed short sutures are useful for microsurgical hepatic artery reconstruction in living-related liver transplantation. Hepatogastroenterology 2000;47:1103-4. [PubMed]
- Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg 1991;214:428-37. [Crossref] [PubMed]
- Wei WI, Lam LK, Ng RW, et al. Microvascular reconstruction of the hepatic artery in live donor liver transplantation: experience across a decade. Arch Surg 2004;139:304-7. [Crossref] [PubMed]
- Miyagi S, Enomoto Y, Sekiguchi S, et al. Microsurgical back wall support suture technique with double needle sutures on hepatic artery reconstruction in living donor liver transplantation. Transplant Proc 2008;40:2521-2. [Crossref] [PubMed]
- Iida T, Kaido T, Yagi S, et al. Hepatic arterial complications in adult living donor liver transplant recipients: a single-center experience of 673 cases. Clin Transplant 2014;28:1025-30. [Crossref] [PubMed]
- Takatsuki M, Chiang YC, Lin TS, et al. Anatomical and technical aspects of hepatic artery reconstruction in living donor liver transplantation. Surgery 2006;140:824-8; discussion 829. [Crossref] [PubMed]
- Julka KD, Lin TS, Chen CL, et al. Reconstructing single hepatic artery with two arterial stumps: biliary complications in pediatric living donor liver transplantation. Pediatr Surg Int 2014;30:39-46. [Crossref] [PubMed]
- Kubota K, Makuuchi M, Takayama T, et al. Simple test on the back table for justifying single hepatic-artery reconstruction in living related liver transplantation. Transplantation 2000;70:696-7. [Crossref] [PubMed]
- Ikegami T, Kawasaki S, Matsunami H, et al. Should all hepatic arterial branches be reconstructed in living-related liver transplantation? Surgery 1996;119:431-6. [Crossref] [PubMed]
- Egawa H, Inomata Y, Uemoto S, et al. Biliary anastomotic complications in 400 living related liver transplantation. World J Surg 2001;25:1300-7. [Crossref] [PubMed]
- Chok KS, Chan SC, Cheung TT, et al. Bile duct anastomotic stricture after adult-to-adult right lobe living donor liver transplantation. Liver Transpl 2011;17:47-52. [Crossref] [PubMed]
- Zafar Zengal M, Zubair F, Zubair B, et al. Immediate Redo Hepatic Artery Reconstruction in Living Donor Liver Transplantation. Available online: https://pdfs.semanticscholar.org/36b9/59b4b3cacde29ecabe770c1b2df8a5601ded.pdf
- Wang CC, Lin TS, Chen CL, et al. Arterial reconstruction in hepatic artery occlusions in adult living donor liver transplantation using gastric vessels. Surgery 2008;143:686-90. [Crossref] [PubMed]
- Albertini A, Lochegnies A, El Khoury G, et al. Use of the right gastroepiploic artery as a coronary artery bypass graft in 307 patients. Cardiovasc Surg 1998;6:419-23. [Crossref] [PubMed]
- Figueras J, Parés D, Aranda H, et al. Results of using the recipient's splenic artery for arterial reconstruction in liver transplantation in 23 patients. Transplantation 1997;64:655-8. [Crossref] [PubMed]
- Lin TS, Yang JC, Chen CL. Hepatic artery reconstruction using radial artery interposition graft in living donor liver transplantation. Transpl Int 2013;26:e28-30. [Crossref] [PubMed]
- Li WF, Lin TS, Chen CL, et al. Using ileocolic artery for successful graft salvage in a recipient with hepatic artery thrombosis after living donor liver transplantation: case report. Transplant Proc 2012;44:581-2. [Crossref] [PubMed]
- Tzeng YS, Hsieh CB, Chen SG. Continuous versus interrupted suture for hepatic artery reconstruction using a loupe in living-donor liver transplantation. Ann Transplant 2011;16:12-5. [Crossref] [PubMed]
- Guarrera JV, Sinha P, Lobritto SJ, et al. Microvascular hepatic artery anastomosis in pediatric segmental liver transplantation: microscope vs. loupe. Transpl Int 2004;17:585-8. [Crossref] [PubMed]
- Marubashi S, Kobayashi S, Wada H, et al. Hepatic artery reconstruction in living donor liver transplantation: risk factor analysis of complication and a role of MDCT scan for detecting anastomotic stricture. World J Surg 2013;37:2671-7. [Crossref] [PubMed]
- Lee CF, Lu JC, Zidan A, et al. Microscope‐assisted hepatic artery reconstruction in adult living donor liver transplantation—A review of 325 consecutive cases in a single center. Clin transplant 2017;31. [Crossref] [PubMed]
- Li PC, Thorat A, Jeng LB, et al. Hepatic artery reconstruction in living donor liver transplantation using surgical loupes: Achieving low rate of hepatic arterial thrombosis in 741 consecutive recipients—tips and tricks to overcome the poor hepatic arterial flow. Liver Transpl 2017;23:887-98. [Crossref] [PubMed]
- Pomposelli JJ. Hepatic Artery Thrombosis (HAT) After Liver Transplant: Not A Surgical Problem? Transplantation 2016. [Epub ahead of print]. [Crossref]
- Eldeen FZ, Roll GR, Derosas C, et al. Pre-operative thromboelastography as a sensitive tool predicting those at risk of developing early hepatic artery thrombosis following adult liver transplantation. Transplantation 2016;100:2382-90. [Crossref] [PubMed]
- Yang Y, Yan LN, Zhao JC, et al. Microsurgical reconstruction of hepatic artery in A-A LDLT: 124 consecutive cases without HAT. World J Gastroenterol 2010;16:2682-8. [Crossref] [PubMed]
- Uchiyama H, Taketomi A, Shirabe K, et al. Microvascular Hepatic Artery Reconstruction in Living Donor Liver Transplantation. In: Abdeldayem H, Allam N. Editor. Liver Transplantation - Technical Issues and Complications. London: IntechOpen, 2012.


