
Surgical outcomes of two-stage hepatectomy for colorectal liver metastasis: comparison to a benchmark procedure
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the western world (1). More than 25% patients with colorectal cancer present with metastatic lesions, and treatment options for metastatic CRC are limited (2).
Chemotherapy alone may assist in symptom reduction and can prolong patients’ survival. However, in order to reach cure, a complete resection of the lesion must be achieved (3-9).
More than 20% of patients may be cured if liver metastases can be completely resected (10). This approach is extremely challenging in cases of multi-lobar liver metastasis, and only 10–20% of these patients are eligible for surgery. A complete resection of metastatic lesions is mandatory for prolonging patient survival (9). However, the preservation of an adequate future liver remnant (FLR) is essential (11-13). Two-stage hepatectomy (TSH) with portal vein embolization (PVE) was introduced almost two decades ago as a proposed technique for the treatment of bi-lobar disease (14). The first stage includes the non-anatomical resection of all left lobe lesions or their ablation by radiofrequency, microwave or irreversible electroporation (IRE). This is followed by PVE performed during or adjacent to surgery. Following the demonstration of sufficient left lobe hypertrophy, a second stage right hepatectomy is performed. The first cases of TSH were introduced by Adam et al., with 13/16 (81.3%) patients completing both stages of the TSH (14). However, a following series demonstrated lower percentages of TSH completion (approximately one third), and a higher associated morbidity and mortality than was previously reported (10,15-17). A primary concern is insufficient FLR following major resections. Recent studies have reported complication rates of 15% in the first stage procedure and 56% in the second stage, with mortality rates of 9–15%, mainly due to liver failure (10,15-17).
Our department has adopted an aggressive approach in the management of CRC liver metastases. We believe that with proper patient selection, TSH can be a safe procedure in patients with bi-lobar disease. The objective of this investigation was to assess the safety of this procedure, by comparing the complication rates of patients who underwent TSH to those who underwent a benchmark operation—a right hepatectomy (RH).
Methods
A retrospective review was performed of all patients who underwent TSH due to multi-lobular liver metastatic CRC in the Hepatobiliary Department in our institution between January 1, 2007 and December 31, 2017. Patients are selected for TSH if it is deemed possible to completely resect/ablate all left-lobe lesions, if the primary colonic tumor is resectable, if no other systemic lesions are present, and if the patient’s general health status permits the performance of this large procedure. All patients undergo a first stage procedure that includes complete metastasectomy from the left lobe (via single or multiple non-anatomical wedge resections) and/or intraoperative ablation (microwave ablation or IRE) of left lobe lesions, followed by a PVE (intraoperatively or adjacent to the procedure). The first aspect of preoperative patient selection included the assessment of the technical ability to clear the left lobe from tumor burden via resection and/or ablative methods. The second aspect assessed the patients’ ability to tolerate the major surgical endeavor. This included strict multidisciplinary evaluation by anesthesiologists, cardiologists, and pulmonologists, with the routine performance of echocardiograms and pulmonary function tests for all patients, and the performance of preoperative cardiac catheterization when deemed necessary by the cardiologist. Each case was discussed preoperatively in a multidisciplinary setting. That, in addition to intraoperative findings (including intraoperative sonographic findings) dictated the decision for ablation versus resection. Our general approach was the performance of non-anatomic resection for peripheral and superficial lesions, while ablation was the modality of choice for smaller and deeper tumors (with the goal of parenchymal preservation) and for tumors that abutted a hepatic vein or portal pedicle that we intended to preserve. Our default institutional ablative method was microwave ablation (MWA), however IRE was utilized for tumors that were adjacent to larger vessels. FLR was subsequently assessed by cross-sectional imaging [computed tomography (CT) or magnetic resonance imaging (MRI)], and, if appropriate, a second stage operation was performed and included a complete right hepatic lobectomy. Patients who underwent TSH were compared to a control group which included patients who underwent a RH as a single procedure for metastatic CRC during the same time period. Patients were admitted and observed overnight in an intensive care setting. Daily monitoring and blood analyses were taken to assess patient status. All patients were followed up 1 month, 3 months, 1 year, and yearly thereafter in our multidisciplinary clinic, and laboratory results (including tumor markers) and imaging studies (CT, MRI, positron-emission tomography) were utilized to rule out recurrence. The study was approved by our institutional ethics committee, and the need for informed consent was waived due to the retrospective nature of the investigation.
Data collected included baseline demographic and preoperative patient characteristics, operative, inter-operative, and postoperative information. The primary outcome of the study was the postoperative complication rate, as graded by the Clavien Dindo classification (17). Major complications were defined as Clavien Dindo ≥3. Secondary outcomes included 90-day mortality, and length of hospital stay.
Statistical analysis was performed using statistical software (SPSS, version 24.0). Univariate analysis with t-test and Chi square was utilized to compare between the study groups. P<0.05 was considered significant for all comparisons.
Results
Patient selection
During the study period, 29 patients underwent TSH, including 13 males and 16 females. Patients’ age ranged from 34 to 77 (mean 57.8±10.3) years. All of these patients had normal preoperative liver function tests, except for three patients with mildly elevated gamma-glutamyl transferase (less than 350 IU/L). None of the patients had preoperative evidence of cirrhosis. Out of these patients, 24 (82.8%) completed the full 2-stage procedure including PVE. Five patients did not proceed to the second stage due to recurrence of disease. In 3 patients, the recurrence was demonstrated on the second procedure upon explorative laparotomy, and the procedure was aborted. In the remaining 2 patients, the recurrence was noted on preoperative cross-sectional imaging, and these patients were excluded from the second procedure. The control group consisted of 35 patients who underwent RH as a sole procedure for CRC liver metastasis, in addition to resection of their primary tumor. This group included 19 males and 16 females, and had an age range of 33–80 (mean 57.8±9.8) years.
Both groups’ pre-, intra-, and interoperative characteristics are summarized in Table 1. It is to be noted that no statistically-significant differences were demonstrated between the groups. However, median preoperative CEA was higher in the TSH than in the RH group (7.8 vs. 3.2 ng/mL, P=0.03).
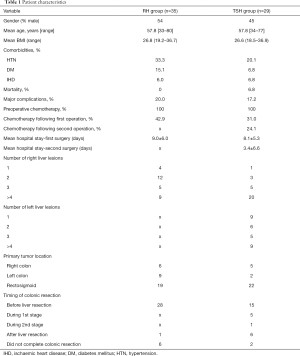
Full table
Operative and interoperative management of the study group
First stage: 29 patients underwent the first stage operation that included the resection of all metastatic lesions from the left liver and/or their ablation using microwave or IRE. Various numbers of liver lesions were encountered and all were completely removed/ablated with no major complications. Three patients underwent anatomical resection of segment 2, five patients underwent anatomical resection of segment 3, three underwent a left lateral segmentectomy, while 13 patients underwent various nonanatomic resections of segments 2, 3 and 4. Mean operative time was 3.2±0.8 hours, and no intraoperative blood transfusion was required. No surgery-related mortality was reported in this first stage.
PVE: all 29 patients underwent PVE (26 patients during the first procedure and 3 in the following 7 days following surgery).
Second stage: 25 out of 29 patients (86%) underwent the second stage operation (RH). Mean operative time among these patients was 4.7±1.0 hours.
Chemotherapy
All of the patients in the TSH cohort received neoadjuvant therapy with variant regimens (Table 2). All patients received preoperative fluoropyrimidine-based chemotherapeutic regimens (FOLFIRI in 17 patients, FOLFOX in 8 patients, and XELOX in 4 patients), with the addition of biologic agents in 14 patients. Nine (31.0%) patients received additional chemotherapy after the first stage and 7 (24.1%) after the second stage. The decision to administer adjuvant chemotherapy was made on a case-to-case basis, following multidisciplinary discussions between oncologic, surgical and radiology staff.
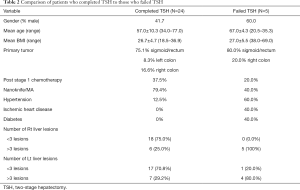
Full table
All of the control group (RH 35 patients) received neoadjuvant therapy with variant protocols, and 15 (42.9%) received postoperative chemotherapy.
Complications
A postoperative major complication rate of 17.2% (5/29) was observed in the TSH group. Two patients (6.9%) were admitted to the intensive care unit (ICU) due to single or multiple organ failure. Four patients (13.8%) required relaparotomy: 2 patients due to anastomotic leak from colonic resection and 2 due to iatrogenic ureteral injury requiring primary repair. One patient presented with a right upper quadrant abscess adjacent to the hepatic resection and underwent CT-guided percutaneous drainage.
The control (RH) group demonstrated a major complication rate of 20.0% (7/35), all of which included intra-abdominal collections requiring percutaneous drainage. Two patients (5.7%) required ICU admission due to single or multi-organ failure.
Mortality
One postoperative mortality occurred in the TSH group (3.4%). This patient was a 59-year-old female who was admitted to the ICU with a multi-organ failure and died from bleeding as a complication of a subclavian catheter insertion. No postoperative (90-day) mortalities occurred in the control group.
Length of stay
Mean length of hospital stay in the TSH cohort was 8.1±5.3 days after the first stage and 3.4±6.6 after the second stage (overall length of hospital-stay 14.7±7.2). The RH cohort demonstrated an overall mean length of stay of 9.0±6.0 days.
Use of MWA or IRE
Intraoperative ablative procedures were utilized in 72.4% (21/29) of the TSH group and in 79.2% (19/24) of the patients who completed the TSH procedure. In 55.2% (16/29), two or more modalities were utilized (microwave ablation/IRE and/or non-anatomical resection). Two of the patients who failed to complete the TSH later underwent ablative modalities.
Comparison between those who failed to complete versus those who completed TSH
Although 29 patients were preoperatively selected for TSH, only 24 (82.8%) successfully completed the two-stage procedure. A detail comparison between patients who completed TSH (24 patients) and those who failed to complete the full TSH (5 patients) is presented in Table 2. It must be noted that the sample sizes are relatively small, and no statistically significant differences were demonstrated between the groups. However, the presence of comorbidities and more than 3 (left and right lobe) lesions was more common in patients who failed TSH.
Long term results
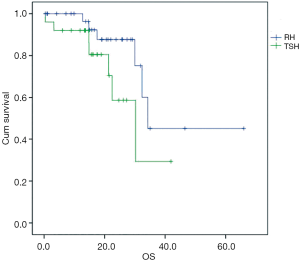
Patients who underwent TSH demonstrated a 1-year survival rate of 70.9%, and a median overall survival of 30.2 months (Figure 1).
Discussion
TSH for CRC metastases was previously associated with high mortality (approximately 7%) and morbidity (ranging from 30% to 60%) (18-23). This current study attempted to demonstrate this procedure’s safety in a carefully-selected cohort of CRC patients with bi-lobar liver metastases, by comparing the procedure’s complications to those of a benchmark single-stage RH. Although it is clear that two inherently different patient groups are being compared, it must be remembered that the objective of this investigation was to demonstrate that when the appropriate patients are selected, TSH is not associated with higher morbidity than RH. Due to the fact that such a comparison has only rarely been reported, this current investigation adds to the available literature (24).
Our study demonstrated lower rates of morbidity and mortality (17.2% and 3.5%, respectively) than those reported in the literature, and comparable complication rates to those who underwent a sole RH (20.0%). The complexity of the procedure and the ability to successfully complete the TSH are contingent on the remaining viable liver tissue and necessity to avoid hepatic insufficiency. Most of the major complications describe previously by the literature were due to liver failure following major tissue resection (14,25).
It is our belief that proper patient selection is vital to the avoidance of this fatal complication. Appropriate patient selection occurs at several levels. A comprehensive multidisciplinary preoperative patient assessment is of extreme importance to decide on the appropriateness of TSH. Patients with multiple bilateral lesions must be strenuously evaluated prior to proceeding to the operation. All patients (5/29) who failed to complete TSH in our cohort presented with bi-lobar disease, with at least 3 lesions in each lobe. Although some patients with similar lesions (29.1%) did complete TSH, it is possible that the presence of more than 3 lesions in each lobe should be considered a relative contraindication for the performance of TSH. This doesn’t necessarily deny eligibility of TSH for patients with more than 3 lesions, however it is clear that these patients must be scrutinized by a multidisciplinary team prior to deciding on surgery. An additional important issue is the assessment of patients’ preoperative general status. In this current cohort, all patients who failed to complete TSH suffered from chronic medical diseases, which may have significantly influenced their ability to recover from this major procedure and avoid postoperative complications. Following the first stage procedure and PVE, the multi-disciplinary assessment should be revisited to rule out disease progression and verify left-sided hypertrophy prior to attempting RH. An additional factor that may influence outcomes is the timing of the colonic resection. In this current investigation, 80.0% of all major complications in the TSH group were related to the concomitant colonic resection and only 20% (n=1) was due to liver insufficiency. It is possible that this is an indication that the colonic resection should be performed separately. In Karoui et al.’s publication, 33 patients designated for TSH underwent colonic resection along with the first-stage procedure (26). Although the authors concluded that the performance of concomitant colonic resections may improve outcomes, reduce the number of procedures and optimize chemotherapy administration, they did report a bowel leak rate of 2/33, which theoretically could have been avoided in the context of TSH by performing the colonic resection in a separate surgery. The timing of colonic resection remains a controversial issue and further investigation on larger numbers of patients is required to reach more definitive conclusions.
We believe that this investigation demonstrates the vital role of ablations in TSH. The high rates of associated morbidity in TSH are largely attributed to the performance of major hepatic resections on a chemotherapy-damaged liver. The utilization of ablative techniques allows hepatic sparing which is commonly not possible when performing resections. They are also generally less time-consuming than resections, and thereby theoretically reduce the “operative trauma”. It is our strong believe that when aiming to perform TSH, when given the option between resection versus ablation, the hepatobiliary surgeon should opt for ablation (if the predicted oncologic outcome of both modalities seems comparable in that specific case). We are convinced that our liberal use of ablative modalities has led to our very low rates of post-TSH hepatic failure. In addition, in cases of necessary large left hepatic resections, we prefer not to perform the PVE in the same surgery and opt to perform it percutaneously a week later.
Imai et al. attempted to identify risk factors for failure of TSH to increase the ability to optimize preoperative patient selection (27). Of their 125 patients planned for TSH, 44 patients (35.2%) could not proceed to the second stage, and their analysis showed that CEA greater than 30 ng/mL, tumor size greater than 4 cm, the use of greater than 12 chemotherapy cycles, and tumor progression under first-line chemotherapy were independent factors for TSH failure.
This investigation has several limitations. Its retrospective nature makes it difficult to completely comprehend the exact decision-making process in patient management. Patients undergoing TSH represent a myriad of different cases, in which a wide variety of therapeutic modalities are attempted, making their uniform analysis challenging. The performance of simultaneous colorectal resection in a portion of the patients further adds to the heterogenicity of the study group. The relatively small patient groups make statistical analysis problematic, and it is possible that with larger patient groups, differences between the cohorts may be more obvious. An additional limitation is that our electronic database lacks certain information, such as documentation of post-PVE volumetric values, presence of intraoperative evidence of chemotherapy-associated liver injury, and occasionally number of chemotherapeutic cycles if administered outside of our institution. Finally, the fact that two oncologically-different patient populations were compared may be considered a flaw of the study. That said, the ultimate objective was to demonstrate the non-inferiority of TSH with regard to postoperative complication rate. Due to paucity of similar reports, we believe that this investigation represents an important addition to the literature.
Conclusions
In carefully-selected patients with bi-lobar CRC liver metastases, TSH seems to be a relatively safe procedure with acceptable morbidity and mortality rates comparable to patients undergoing sole RH. Liberal use of ablative techniques for left lobe clearance is recommended, in order to reduce operative trauma and maximize FLR. Factor such as number of lesions, comorbidities and the timing of colonic resection should be considered and evaluated in order to improve the outcomes of the procedure.
Acknowledgements
The authors would like to acknowledge Gal Schtrechman for her assistance in the statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional ethics committee, and the need for informed consent was waived due to the retrospective nature of the investigation.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018;4:1553-68. [Crossref] [PubMed]
- Littlejohns P, Tamber S, Ranson P, et al. Treatment for liver metastases from colorectal cancer. Lancet Oncol 2005;6:73. [Crossref] [PubMed]
- Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984;119:647-51. [Crossref] [PubMed]
- Fortner JG, Silva JS, Golbey RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg 1984;199:306-16. [Crossref] [PubMed]
- Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Registry of Hepatic Metastases. Surgery 1988;103:278-88. [PubMed]
- Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 1989;210:127-38. [Crossref] [PubMed]
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. [Crossref] [PubMed]
- Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Association Francaise de Chirurgie. Br J Surg 1997;84:977-80. [Crossref] [PubMed]
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241-6. [Crossref] [PubMed]
- Tanaka K, Shimada H, Matsuo K, et al. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol 2007;33:329-35. [Crossref] [PubMed]
- Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 2003;10:1059-69. [Crossref] [PubMed]
- Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000;231:480-6. [Crossref] [PubMed]
- Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208-17. [Crossref] [PubMed]
- Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85. [Crossref] [PubMed]
- Maki H, Satou S, Nakajima K, et al. Two-stage hepatectomy aiming for the development of intrahepatic venous collaterals for multiple colorectal liver metastases. Surg Case Rep 2018;4:17. [Crossref] [PubMed]
- Kochbati S, Ben Miled M, Boussema F, et al. Leflunomide in the treatment of rheumatoid arthritis. Tunis Med 2005;83:91-7. [PubMed]
- Pamecha V, Nedjat-Shokouhi B, Gurusamy K, et al. Prospective evaluation of two-stage hepatectomy combined with selective portal vein embolisation and systemic chemotherapy for patients with unresectable bilobar colorectal liver metastases. Dig Surg 2008;25:387-93. [Crossref] [PubMed]
- Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083-90. [Crossref] [PubMed]
- Faitot F, Soubrane O, Wendum D, et al. Feasibility and survival of 2-stage hepatectomy for colorectal metastases: definition of a simple and early clinicopathologic predicting score. Surgery 2015;157:444-53. [Crossref] [PubMed]
- Muratore A, Zimmitti G, Ribero D, et al. Chemotherapy between the first and second stages of a two-stage hepatectomy for colorectal liver metastases: should we routinely recommend it? Ann Surg Oncol 2012;19:1310-5. [Crossref] [PubMed]
- Narita M, Oussoultzoglou E, Jaeck D, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg 2011;98:1463-75. [Crossref] [PubMed]
- Tsai S, Marques HP, de Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010;12:262-9. [Crossref] [PubMed]
- Turrini O, Ewald J, Viret F, et al. Two-stage hepatectomy: who will not jump over the second hurdle? Eur J Surg Oncol 2012;38:266-73. [Crossref] [PubMed]
- Schadde E, Slankamenac K, Breitenstein S, et al. Are two-stage hepatectomies associated with more complications than one-stage procedures? HPB (Oxford) 2013;15:411-7. [Crossref] [PubMed]
- Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg 2000;231:743-51. [Crossref] [PubMed]
- Karoui M, Vigano L, Goyer P, et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg 2010;97:1354-62. [Crossref] [PubMed]
- Imai K, Benitez CC, Allard MA, et al. Failure to Achieve a 2-Stage Hepatectomy for Colorectal Liver Metastases: How to Prevent It? Ann Surg 2015;262:772-8; discussion 778-9. [Crossref] [PubMed]


