
The impact of alloantibodies directed against the second donor on long-term outcomes of repeat liver transplantation
Introduction
Conventionally, liver allografts have been considered to be relatively resilient to allogeneic immunological attack compared to kidney or pancreas allografts. Liver transplants are usually allocated according to blood group (ABO) compatibility but not according to human leucocyte antigen (HLA) crossmatch or presence of preformed donor-specific antibodies (DSA). However, it was recently found that the previously claimed liver protective effect on kidney graft in simultaneous liver-kidney transplant was not complete, especially in the presence of preformed class II DSA (1,2). When sensitive solid phase–based anti-HLA antibody detection methods became available, the role of donor specific antibodies, either preformed before liver transplantation, or de novo (generated after liver transplantation), was revisited in many studies (3-15). Some supported detrimental effects of DSA or positive cross match on graft or patient survival (6-10), but others failed to find significance (11-15). The reason for uncertainty in cohort studies may be the low prevalence of the study factor in the test population. Preformed DSA is usually rare (~10%) in candidates for their first liver transplantation. Of the mechanisms to develop anti-HLA antibodies, prior exposure to alloantigen by transplantation sensitizes patients more effectively than blood transfusion or pregnancy (16). As a consequence, the frequency of preformed DSA should be higher in candidates for a second liver transplantation than for the initial transplantation, making them a better group in whom the impact of DSA could be studied. In order to determine if preformed DSA affects liver graft outcomes, we performed a retrospective cohort study of consecutive patients who received a second liver transplantation in order to determine if the prevalence of DSA was higher after the first transplant and then to compare graft and patient survival in recipients who had DSA to the second donor (D2SA+) before retransplantation to those who did not have DSA to the second donor (D2SA−).
Methods
We reviewed all second liver transplantations between 1990 and 2014 at University Hospital of London Health Science Centre (LHSC), London, Ontario, Canada. Illness severity, ABO compatibility and size-matching, but not recipient-donor cross-matching or HLA matching, were used to allocate liver grafts to candidates on the waiting list for repeat liver transplantation. All patients undergoing a second liver transplantation whose pre-operative serum and donor HLA typing were available were included in the study. We excluded ABO incompatible transplants, recipients of multiple organs, and transplants from living donors or donors after cardiac death (DCD). This study was approved by institutional ethical review committee (University of Western Ontario Research Ethics Board protocol #106961).
Patient blood samples were collected immediately before retransplantation and stored at the Transplant Immunology Lab, LHSC. Blood samples were screened with multiple-antigen coated Luminex PRA beads (One Lambda, Canoga Park CA) to determine the presence of anti-HLA antibodies. Samples with positive antibodies were tested with Luminex single antigen beads (SAB) (One Lambda, Canoga Park CA) for antibodies specificities. If not specifically defined, positive reactions were called if median fluoresce intensity (MFI) was more than 1,000 and antibody profile made sense according to cross reactivity and/or epitope analysis. Sensitivity studies included analysis using MFI over 10,000 (10k) as cut-off for D2SA+. D2SA status was determined with full donor typing for HLA-A, B, C, DRB1, DRB3/4/5, DQA1/B1 and DPA1/B1 in a low to intermediate resolution reverse sequence-specific oligo (SSO) probe LabType kit (One Lambda, Canoga Park CA). Sum MFI for D2SA were calculated by adding MFIs for each specific D2SA.
Clinical outcomes were collected by chart review and extensive queries for follow-up until April 2018. Recipient and graft survival curves were plotted using Kaplan-Meier method and analyzed for statistical significance using the Log-Rank test. Baseline characteristics were compared between the two groups of patients using the Chi-squared test or Fisher’s exact test for categorical variables, two-tailed t-test for normally distributed continuous variables. Hazard ratios were determined using cox proportional hazards model for either univariate or multivariate analysis. All statistical analyses were done with IBM SPSS version 25.
Results
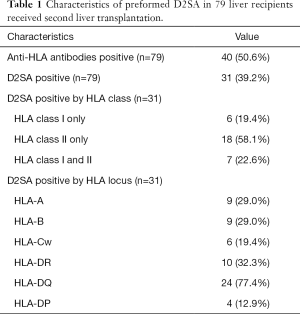
We identified 84 recipients of a second liver transplant for whom pre-retransplantation sera and donor HLA typing were available. Five patients were excluded from the study: four because they received another organ transplant with either the first or second liver transplant and one patient because incomplete medical information. In the end 79 patients who received second liver transplant from 1991 to 2014 were included in this study. As shown in Table 1, about half (50.6%) of the patients waiting for a second liver transplant were found to be positive for anti-HLA antibodies. Preformed D2SA was identified in 31 (39.2%) patients. D2SA was directed to HLA class 1 only in 6 (19.4%) patients, to HLA class II only in 18 (58.1%) patients, to both HLA class I and class II in 7 (22.6%) patients. Anti-HLA-DQ D2SA were present in majority (24, 77.4%) of D2SA positive patients.
Full table
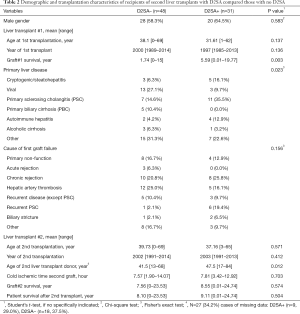
Most demographic and baseline characteristics for D2SA+ subgroup and D2SA− subgroup were found to be very similar (Table 2). Survival of the first liver graft was significantly (P=0.003) shorter in D2SA− patients [1.74 (range, 0–15) years] than in the D2SA+ cohort [5.59 (range, 0.01–19.77) years]. Components of primary liver diseases are marginally different in the two subgroups with notably more primary sclerosing cholangitis (PSC) and less viral diseases in D2SA+ group than in D2SA− group. The age of the second donor was slightly older (47.5 years) in D2SA+ patients than in D2SA− patients (41.5 years) but this field was empty in 34% of the retransplantation database.
Full table
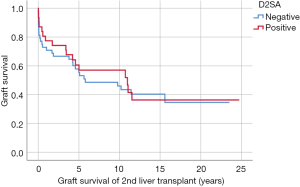
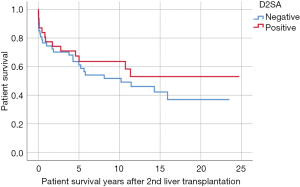
During this study period, 45/79 (57.0%) second liver grafts were lost; 40/79 (49.4%) patients died; and 12/79 (15.2%) patient received a third liver transplant. Mean survival of the second graft was similar in D2SA+ and D2SA− cohorts [8.55 (range, 0.01–24.74) vs. 7.56 (range, 0–23.53) years respectively, P=0.574]. Mean patient survival after second liver transplantation was similar in D2SA+ and D2SA− cohorts [9.11 (range, 0.01–24.74) vs. 8.10 (range, 0–23.53) years respectively, P=0.504]. Kaplan-Meier survival for D2SA+ versus D2SA− subgroups are shown in Figures 1,2. No statistically differences were found for either survival of second liver graft (P=0.724) or patient survival after second liver transplant (P=0.328).
Univariate cox proportional hazard regression of the different types and strengths of D2SA on the outcome the second liver transplant are summarized in Table 3. Presence of D2SA to either class I, class II or specific loci of HLA- A, -B, -Cw, -DR, -DQ, -DP were not found to be associated with increased risk for graft loss. Additive strengths of D2SA (indicated by sum MFI >10k, >20k, >40k, >100k) were not relevant to outcome. MFI >10k for class I D2SA or class II D2SA were not associated with graft loss. Presence of either DR or DQ D2SA was not a statistically or clinically significant cause of graft loss (HR =1.196, P=0.565). In multivariate analysis adjusting risks from age, gender, cold ischemia time, primary diseases, causes of loss and survival years of 1st graft, neither class I D2DSA (HR =1.101, P=0.92) nor class II D2SA (HR =1.74, P=0.359) were found to be significant risk factors for graft survival.
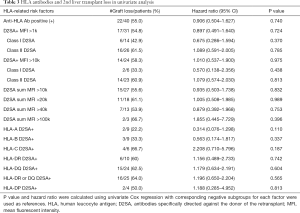
Full table
Discussion
Liver retransplantations were often excluded from studies due to substantially worse outcome than 1st liver transplant. However, we believed this group might be a better cohort to study the impact of DSA because of an expected higher prevalence of DSA. This was confirmed when we identified anti-HLA antibodies in approximately half the cohort and these antibodies were directed against the second graft in 40%. Interestingly, D2SA+ recipients retained their initial graft much longer than D2SA− recipients, suggesting that the length of exposure to the alloantigens played a role in the development of antibody.
Goh and colleagues studied the outcome of 118 liver retransplants and found class I anti-HLA antibodies but not DSA to be associated with inferior graft survival (17). The MFI of the DSA was not reported in their paper but most (97.5%) cases were transplanted with negative CDC cross matches. The study reported here is the first impact study of preformed DSA, determined using modern HLA detecting methods specific to the 2nd donor, on the long-term outcome of liver re-transplantation. The cohort underwent transplantation without knowledge of crossmatch or DSA status. In order to remove heterogenous risk of an adverse outcome, we excluded transplantations from living donors, DCD, ABO incompatible donors and combined transplants. Anti-HLA antibodies were batch tested using modern sensitive solid phase methods. The D2SA+ and D2SA− groups were equivalent in size, making comparison efficient. We could find no signal related to the presence of D2SA in the long-term survival of 79 retransplants. We did not find a clinically or statistically significant detrimental effect of preformed D2SA to either graft or patient survival. Therefore, our results are compatible with the results described by Goh and colleagues.
We did find that patients who lost their first liver transplant from acute rejection were more likely to lose the second liver transplant (HR =6.1, P=0.024) in multivariate analysis. Different deleterious roles of class II rather than class I DSA are thought to occur in liver transplantation either because class I DSA are more easily absorbed by liver (6,12) or because of the presence of protective IgM antibodies (18). However, we were unable to find an effect if we studied D2SA type (HLA-A, B, C, DRB1, DRB3/4/5, DQA1/B1 and DPA1/B1). While allogenic memory plays important role in the loss of liver transplants, it may be that cellular rather humoral responses predominate.
The sample size of this study is small. High D2SA prevalence and the matching size of the test groups mitigates this limitation to some extent. We think the group sizes would have been sufficient to see the effect of D2SA on graft outcome if kidney rather than liver retransplantation was studied. This study, combined with the uncertainty remaining from the many studies of primary liver transplantation (6-15), supports the convention that liver transplantation is less prone to humoral immunological loss than kidney transplantation. The reasons for this difference are not known.
One mechanism to consider is immunoglobulin (Ig) class switch (19). Liver grafts have been shown to produce anti-donor IgM, where preformed IgM has been shown to be either irrelevant or protective in kidney transplantation (18). So far studies of IgG subclasses give tantalizing hints that the immune response is deviated to the ‘type 2’ variety which is exploited by pregnancy or parasites to avoid rejection (20). However, type 2 immune response deviation may leave the graft open for chronic rejection. Against this hypothesis is the finding, reported by O’Leary and colleagues, that the presence of complement fixing DSA (a type 1 humoral response) at one year after transplantation, is associated with an increased risk of chronic rejection of the graft (21). Some of the patients in this study were followed for over 20 years. We found mean graft survivals of 7.56 (range, 0–23.53) years in the D2SA− recipients vs. 8.55 (range, 0.01–24.74) years (P=0.574) in the D2SA+ group. It may be that either other competing risk factors or incomplete effects of D2SA are at play preventing a signal from being apparent. Ultra-long, fully powered studies will be required to dissect the various processes at work. Antibody titer or complement fixation may be better measures than MFI (22). Innovative targets such as HLA, allele and eplet mismatching may unlock some of the mystery (23).
We did not include the types of immunosuppression taken by the patients in our study. Meta-analysis shows a small but significant difference in graft survival between cyclosporine and tacrolimus in primary liver transplantation (24). We used cyclosporine in the early years of the study and later tacrolimus. Patients were not routinely switched from one calcineurin inhibitor to the other and we did not differentiate between primary transplantation or repeat transplantation. No era difference (P=0.412) was seen between the D2SA− group (median 2002, 1991–2014) and the D2SA+ group (median 2003, 1991–2013). Variances in immunosuppressant and surgical technique during the long study period are unlikely contribute to our findings.
We commenced this study in the belief that the low prevalence of DSA before primary liver transplantation was the reason that previous studies have failed to prove the deleterious effect of DSA. If such a deleterious effect was shown, strategies to mitigate this effect would be required. We were able to see a high prevalence of DSA in candidates for liver retransplantation proving the fact that liver transplantation does sensitize the recipient. However, we were unable to show a deleterious effect of these antibodies, either as a whole or of a specific type. The current practice of allocating liver grafts without regard to DSA remains valid. However, programs are encouraged to apply laboratory tools currently used for kidney transplantation to unlock the secrets of how the liver modifies the immune response or remains resilient to it. The reward for understanding these phenomena will not only apply to liver transplantation but may help us understand and combat primary and metastatic liver cancer too.
Acknowledgments
The authors sincerely thank members of transplant Immunology Lab for help on HLA data collection.
Footnote
Conflicts of Interest: Presented at American Transplant Congress 2018, Seattle, WA, USA. Data sharing: Anonymized subject level data will be available. Registered with www.clinicaltrials.gov NCT03815864.
Ethical Statement: This study was approved by institutional ethical review committee (University of Western Ontario Research Ethics Board protocol #106961).
References
- O'Leary JG, Gebel HM, Ruiz R, et al. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant 2013;13:954-60. [Crossref] [PubMed]
- Levitsky J, O'Leary JG, Asrani S, et al. Protecting the Kidney in Liver Transplant Recipients: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 2016;16:2532-44. [Crossref] [PubMed]
- Taner T, Stegall MD, Heimbach JK. Antibody-mediated rejection in liver transplantation: current controversies and future directions. Liver Transpl 2014;20:514-27. [Crossref] [PubMed]
- O'Leary JG, Demetris AJ, Friedman LS, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant 2014;14:779-87. [Crossref] [PubMed]
- Bruneval P, Angelini A, Miller D, et al. The XIIIth Banff Conference on Allograft Pathology: The Banff 2015 Heart Meeting Report: Improving Antibody-Mediated Rejection Diagnostics: Strengths, Unmet Needs, and Future Directions. Am J Transplant 2017;17:42-53. [Crossref] [PubMed]
- O'Leary JG, Kaneku H, Jennings LW, et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl 2013;19:973-80. [Crossref] [PubMed]
- O'Leary JG, Kaneku H, Banuelos N, et al. Impact of IgG3 subclass and C1q-fixing donor-specific HLA alloantibodies on rejection and survival in liver transplantation. Am J Transplant 2015;15:1003-13. [Crossref] [PubMed]
- Kaneku H, O'Leary JG, Banuelos N, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant 2013;13:1541-8. [Crossref] [PubMed]
- den Dulk AC, Shi X, Verhoeven CJ, et al. Donor-specific anti-HLA antibodies are not associated with nonanastomotic biliary strictures but both are independent risk factors for graft loss after liver transplantation. Clin Transplant 2018;32:e13163. [Crossref] [PubMed]
- Levitsky J, Kaneku H, Jie C, et al. Donor-Specific HLA Antibodies in Living Versus Deceased Donor Liver Transplant Recipients. Am J Transplant 2016;16:2437-44. [Crossref] [PubMed]
- Del Bello A, Congy-Jolivet N, Danjoux M, et al. De novo donor-specific anti-HLA antibodies mediated rejection in liver-transplant patients. Transpl Int 2015;28:1371-82. [Crossref] [PubMed]
- Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant 2012;12:1504-10. [Crossref] [PubMed]
- McCaughan JA, Robertson V, Falconer SJ, et al. Preformed donor-specific HLA antibodies are associated with increased risk of early mortality after liver transplantation. Clin Transplant 2016;30:1538-44. [Crossref] [PubMed]
- Lunz J, Ruppert KM, Cajaiba MM, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: can C4d stains help in monitoring? Am J Transplant 2012;12:171-82. [Crossref] [PubMed]
- Ruiz R, Tomiyama K, Campsen J, et al. Implications of a positive crossmatch in liver transplantation: a 20-year review. Liver Transpl 2012;18:455-60. [Crossref] [PubMed]
- Tambur AR, Campbell P, Claas FH, et al. Sensitization in Transplantation: Assessment of Risk (STAR) 2017 Working Group Meeting Report. Am J Transplant 2018;18:1604-14. [Crossref] [PubMed]
- Goh A, Scalamogna M, De Feo T, et al. Human leukocyte antigen crossmatch testing is important for liver retransplantation. Liver Transpl 2010;16:308-13. [Crossref] [PubMed]
- McAlister CC, Gao ZH, McAlister VC, et al. Protective anti-donor IgM production after crossmatch positive liver-kidney transplantation. Liver Transpl 2004;10:315-9. [Crossref] [PubMed]
- McAlister VC. Anti-donor immunoglobulin G subclass in liver transplantation. HepatoBiliary Surg Nutr 2019;8:125-8. [Crossref] [PubMed]
- Gao ZH, McAlister VC, Wright JR Jr, et al. Immunoglobulin-G subclass antidonor reactivity in transplant recipients. Liver Transpl 2004;10:1055-9. [Crossref] [PubMed]
- O'Leary JG, Smith C, Cai J, et al. Chronic AMR in Liver Transplant: Validation of the 1-Year cAMR Score's Ability to Determine Long-term Outcome. Transplantation 2017;101:2062-70. [Crossref] [PubMed]
- Tambur AR, Herrera ND, Haarberg KM, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant 2015;15:2421-30. [Crossref] [PubMed]
- Forner D, Liwski R, Alwayn I. Human leukocyte antigen, allele, and eplet mismatches in liver transplantation; observations from a small, single center cohort. Hum Immunol 2018;79:154-9. [Crossref] [PubMed]
- McAlister VC, Haddad E, Renouf E, et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant 2006;6:1578-85. [Crossref] [PubMed]


