
One checkpoint may hide another: inhibiting the TGFβ signaling pathway enhances immune checkpoint blockade
Tumors employ several mechanisms to evade recognition by the immune system. Among these are epigenetic changes such as loss of the antigen presenting machinery (e.g., HLA-A,B,C) that cause tumors to become invisible to the immune system. They can employ mechanisms to suppress the immune response through the enzyme indoleamine dioxygenase (IDO) and the inhibitory receptor programmed death ligand 1 (PD-L1). Both tumor cells and the surrounding stroma can be the source of such molecules. Tumors can recruit suppressive immune cells [regulatory T cells (Tregs), myeloid derived suppressor cells (MDSCs)] that inhibit T cell responses to tumors mainly by secreting anti-inflammatory cytokines (1,2). TGFβ is the most well characterized of these cytokines. TGFβ is a pleotropic cytokine that is important in regulating immune responses. It is found in many cancer types and is associated with poor clinical outcome (3). Depending on the context of the microenvironment, TGFβ can suppress the immune system by inducing Tregs, and inhibiting CD4+ and CD8+ T cell effector functions such as their activation or cytolytic activity. There are three isoforms of TGFβ in humans and mice (TGFβ1, TGFβ2 and TGFβ3) that have high homology with each other. Numerous cells make and secrete the inactive latent form of TGFβ into the extracellular space; however, only a few cells within the tumor microenvironment (TME) contain the machinery (αv integrins, proteases, GARP etc.) to convert it to its active form. It appears that the suppressive functions of TGFβ are mediated not only by its synthesis but also by the extent of its activation (4). Aside from its role in cancer, TGFβ plays a critical role in controlling aberrant immune responses against self, such as in autoimmune diseases.
Since the FDA approval of Ipilimumab in 2011 and Pembrolizumab in 2014, immunotherapy has moved to the forefront of cancer care. While we have seen promising success of these agents as monotherapies, it is increasingly evident that the combination of these agents (e.g., Ipilumumab and Nivolumab) together or with other conventional therapies [e.g., chemotherapy and radiation therapy (RT)] and novel immunotherapy targets will provide greater clinical benefit. However, rational design of combination therapy is necessary to enhance the efficacy of these immunotherapies. When it comes to targeting specific molecules with immunotherapy, it is imperative to understand the spatial and temporal expression of these molecules in the tumor microenvironment. Secreted factors such as TGFβ can be made by tumor cells, stromal fibroblasts and immune infiltrates. It is important to determine which cells predominantly express the target molecule. Another level of complexity comes from the fact that not all cancer types contain the same components of the microenvironment. Furthermore, the timing and doses of agents to be combined will need to be optimized. Some combinations have already been shown to be counterproductive due to inefficient timing (5). It is imperative to understand the biology of the immune system and the TME in order to design rational hypothesis-driven preclinical and clinical trials of combination therapy.
Two studies by Mariathasan et al. (6) and Tauriello et al. (7) in Nature highlighted how understanding the mechanisms behind the failure of immune checkpoint blockade in urothelial and colorectal cancer (CRC) can aid in developing treatment to overcome resistance to immunotherapies. It is now recognized that immune checkpoint blockade works best in tumors that are heavily infiltrated by T cells or “hot” tumors. Conversely, immunotherapies are less effective in tumors that either lack T cells, referred to as “immune desert” or “cold” tumors, or in tumors that exclude T cells. Mariathasan et al. and Tauriello et al. showed that the presence of TGFβ in the stroma of tumors leads to exclusion of T cell infiltration into tumors by trapping them in the periphery. Concurrent blockade of TGFβ signaling and PD-L1 in these immune excluded tumors lead to greater anti-tumor effects by turning an immune excluded tumor into an inflamed “hot” tumor (Figure 1). In addition, it appears that CD8+ T cells were necessary for the anti-tumor efficacy as both studies show enhanced CD8+ T cell activation.
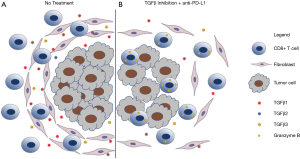
Tauriello et al., modeled microsatellite-stable (MSS) human CRC using genetically engineered mice (designated LAKTP), which developed carcinomas that displayed a TME with high levels of stromal TGFβ activity conferring poor prognoses (3,8). In this model, cancer associated fibroblasts (CAFs) were the main source of TGFβ in mouse and human CRC samples. Treatment with galunisertib, a TGFBR1-specific inhibitor, reduced primary tumors and metastatic disease burden. The addition of anti-PD-L1 with galunisertib eradicated most overt metastatic disease and prolonged overall survival for more than a year after treatment. Activation of both CD4+ and CD8+ T cells was observed with galunisertib treatment. A complementary study by Mariathasan et al. evaluated biomarkers of clinical responses to the anti-PD-L1 antibody, atezolizumab, in patients with metastatic urothelial cancer. Transcriptomic evaluation revealed that clinical response was associated with a CD8+ effector T cell phenotype and high tumor mutation burden, but TGFβ signaling in fibroblasts was associated with poor response and survival (3). This finding was particularly relevant for the T cell excluded subtype of metastatic urothelial cancer, where a pan-fibroblast TGFβ response signature (F-TBRS) was significantly associated with non-responders.
Both studies above (Tauriello et al. and Mariathasan et al.) suggest that targeting the TME through inhibition of TGFβ signaling may be a means to enhance checkpoint blockade immunotherapy across several cancer types. In both cases, the authors showed that the source of TGFβ is primarily derived from CAFs, which express high levels of TGFβ1 and TGFβ3. To date, most preclinical studies involving the TGFβ pathway use tumor models that are enriched in stromal components such as CAFs. However, the role and source of TGFβ in stroma-poor tumors, such as melanoma, remains to be investigated. It will be interesting in the future to determine whether TGFβ blockade will only benefit patients whose tumors are enriched with CAFs. In addition, TGFβ has been shown to have profound effects on suppressing both adaptive and innate immune cells. Most studies on TGFβ focus on its immunosuppressive role on CD4+ and CD8+ T cells (9). These two studies demonstrated that TGFβ inhibition enhanced CD4+ and CD8+ T cell activation and differentiation, especially when combined with PD-L1 blockade. However, the authors did not explore the effects of TGFβ inhibition on innate immune cells. It is well known that TGFβ can promote immunosuppressive macrophages and tolerogenic dendritic cells (9). It would be interesting to see the effects of TGFβ inhibition on innate immune cell populations in the tumor microenvironment and the periphery.
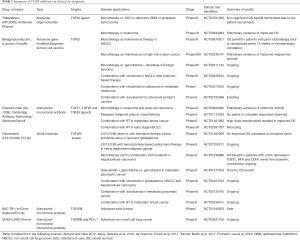
TGFβ signaling blockade can be achieved by targeting discrete steps in the processing of TGFβ and its signaling pathway: ligand biosynthesis, ligand-receptor interaction, and downstream signal transduction. To target ligand production, antisense oligonucleotides and antisense RNA methods are currently being tested (10,11). The ligand-receptor interaction can be interrupted using TGFβ neutralizing monoclonal antibodies and soluble receptors. The most developed of these include Fresolimumab, which has pan-TGFβ ligand blocking activity, LY2382770, a TGFβ1 ligand-selective blocking antibody, and IMC-TR1, a TGFβRII-blocking antibody. Of note, αv integrins (αvβ6 and αvβ8) were shown to be involved in the activation of TGFβ (4). There is now an anti-β6 integrin antibody in clinical development (10,12). Galunisertib (LY2157299) is a small molecule inhibitor of TGFβRI and is the most developed TGFβ receptor kinase inhibitor (10,11). Lastly, M7824 is a fusion protein composed of the extracellular domain of human TGFβRII, acting as a TGFβ trap, connected to the C-terminus of human anti-PD-L1 (13). So far, the most advanced inhibitors in the clinic target TGFβ signaling directly or block all three isoforms of TGFβ. Given the homeostatic roles of TGFβ, including proliferation, differentiation, and migration, inhibition of all 3 isoforms may be detrimental to normal physiology in treated individuals. In fact, there has been side effects and toxicities associated with TGFβ inhibitors in the past; therefore, selective inhibition of one isoform rather than pan-TGFβ blockade may lead to adequate tumor control while reducing off target effects. Currently, isoform specific inhibitors of TGFβ1 and TGFβ3 are undergoing clinical development (14). Table 1 summarizes the disease indications and the stage of development each therapy discussed above has reached. So far, there is no striking efficacy observed with these agents as monotherapies; however, a vast amount of pre-clinical data exists to support their combination with other agents.
Full table
As other forms of anti-cancer treatment impact TGFβ production and activity, rational combination therapies can be crafted. RT can activate TGFβ through reactive oxygen species, which in turn leads to radioresistance and dose-limiting toxicities. The sustained therapeutic effects of RT can be further enhanced by combining it with anti-TGFβ therapy to enhance tumor cytotoxicity, even outside the field of radiation (abscopal effect), while reducing associated toxicities of both therapies (15). Similarly, the studies by Tauriello et al. and Mariathasan et al. have revived the application of TGFβ inhibition in cancer and provided pre-clinical and clinical rationale for testing the combination of TGFβ inhibitors with PD-1/PD-L1 blockade in various cancers.
Acknowledgments
Funding: This study was supported by NIH grants R01CA056821, P01CA33049, and P01CA59350, and MSKCC Cancer Center Core Grant P30CA008748. This study was supported by funding from Ludwig Institute for Cancer Research, Swim Across America, Parker Institute for Cancer Immunotherapy, Howard Hughes Medical Institute and the Breast Cancer Research Foundation.
Footnote
Conflicts of Interest: T Merghoub is a consultant for: Immunos Therapeutics and Pfizer; is co-founder and has equity in: IMVAQ therapeutics; has research support from: Bristol-Myers Squibb, Surface Oncology, Kyn Therapeutics, Infinity Pharmaceuticals, Inc., Peregrine Pharmaceuticals, Inc., Adaptive Biotechnologies, Leap Therapeutics, Inc., and Aprea. is an inventor on patent applications related to work on Oncolytic Viral therapy, Alpha Virus Based Vaccine, Neo Antigen Modeling, CD40, GITR, OX40, PD-1 and CTLA-4.
References
- Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer 2010;127:759-67. [PubMed]
- Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol 2011;90:31-6. [Crossref] [PubMed]
- Bald T, Smyth MJ. TGFbeta shuts the door on T cells. Br J Cancer 2018;119:1-3. [Crossref] [PubMed]
- Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol 2014;32:51-82. [Crossref] [PubMed]
- Messenheimer DJ, Jensen SM, Afentoulis ME, et al. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin Cancer Res 2017;23:6165-77. [Crossref] [PubMed]
- Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544-8. [Crossref] [PubMed]
- Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018;554:538-43. [Crossref] [PubMed]
- Romero D. Immunotherapy: Inhibition of TGFbeta enhances immune-checkpoint blockade. Nat Rev Clin Oncol 2018;15:201. [Crossref] [PubMed]
- Dahmani A, Delisle JS. TGF-beta in T Cell Biology: Implications for Cancer Immunotherapy. Cancers (Basel) 2018;10:194. [Crossref] [PubMed]
- Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 2012;11:790-811. [Crossref] [PubMed]
- Neuzillet C, Tijeras-Raballand A, Cohen R, et al. Targeting the TGFbeta pathway for cancer therapy. Pharmacol Ther 2015;147:22-31. [Crossref] [PubMed]
- de Gramont A, Faivre S, Raymond E. Novel TGF-beta inhibitors ready for prime time in onco-immunology. Oncoimmunology 2016;6:e1257453. [Crossref] [PubMed]
- Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med 2018.10. [PubMed]
- Gupta A, Budhu S, Giese R, et al. Abstract 4716: Targeting specific TGF-β isoforms in combination with radiation therapy leads to differential antitumor effects in mouse models of cancer. Cancer Res 2018.78. abstract 4716.
- Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res 2015;75:2232-42. [Crossref] [PubMed]


