Development and validation of a nomogram for survival benefit of lymphadenectomy in resected gallbladder cancer
Introduction
Gallbladder cancer (GBC) is one of the most common biliary tract malignancies (1,2). It presents with a low annual incidence (3), poor prognosis (1,3,4) and high mortality, because of a high proportion of early lymph node (LN) metastases (5). LN status plays an important role in prognosis (6-8), i.e., positive LN status indicates a poor prognosis. Surgery remains the first line therapy for patients with resected GBC (9). There is much variation in literatures about what composes a “radical cholecystectomy” and/or an “appropriate lymphadenectomy” (10). For complete resection, extended surgical procedures, such as major hepatectomy and adequate extensive lymphadenectomy, even common bile duct resection or pancreatoduodenectomy, are often required (11). Although lymphadenectomy enables to remove more regional LNs and facilitate accurate staging of cancer, it also increases the operative difficulty and risk. Furthermore, even after extensive lymphadenectomy, not all patients with GBC can benefit from it. Therefore, whether lymphadenectomy with more regional LNs for a risk of metastatic disease could enhance or contribute to this curative potential remains debated and unproven.
Several different LN staging/scoring systems, such as tumor-node-metastasis, LN ratio, the log odds of positive LN, have been proposed to stratify the prognosis of patients with GBC. Unfortunately, none of them focuses on this debate. Moreover, it is not easy and convenient to make decision on who need to be performed with more regional lymphadenectomy during operation, according to the results of frozen-sections alone. Due to the rarity of GBC and the lack of large-scale prospective randomized clinical trials, the actual benefit for removing more and/or less regional LNs at risk of GBC has not been well established. As a result, there is little evidence for clinicians to rely on to determine which patients will obtain benefit from more regional LNs.
The primary aim of this study was to create a decision model to estimate individualized potential survival benefit of lymphadenectomy with more and/or less regional LNs for patients with resected GBC.
Methods
Study population
Surveillance, Epidemiology and End Results (SEER) database, which covers approximately 26% of the U.S. population, provides patients’ data, including patient demographics, tumor morphology, staging, treatment detail, follow-up and so on. Patients who underwent resection for GBC between 2004 and 2014 were identified in the SEER database of the National Cancer Institute. GBC was identified using the International Classification of Diseases (ICD-O-3) (C23.9) codes, and patients diagnosed at autopsy, or none of regional LNs removed were excluded. Standard patient demographic and clinicopathologic data, including size, grade, and histological stage, was collected. In addition, one to three regional LNs removed was defined as “lymphadenectomy with less regional LNs”, while four or more regional LNs removed was defined as “lymphadenectomy with more regional LNs”. Patients who underwent resection for GBC from January 2007 to December 2012 at author’s institution were included in the study as external validation data.
Statistical analysis
Statistical analyses were performed using the SPSS 24.0 or R software packages. The primary end point of interest in this study was overall survival (OS). Observed covariates were age, sex, race, grade, tumor size, American Joint Commotion Cancer of T stage according to 7th edition and receipt of non-primary surgery and so on. A propensity score 1:1 matching method was performed to balance observed covariates in two groups using the SPSS 24.0. By assigning propensity score weights to each patient and incorporating these weights into model construction, we can reduce inherent biases in retrospective non-randomized regression analyses. Multivariate regression survival analysis was performed to identify significant factors. Then, two survival modeling methods such as Semiparametric model (Cox proportional hazards) and accelerated failure time parametric model (lognormal) were compared using Akaike’s Information Criterion. The best model was selected and tested by the internal data form SEER database and external validation data from authors’ hospital using both discrimination and calibration. Discrimination was evaluated using the Harrell’s concordance index (C-index). Calibration, which compares predicted with actual survival, was evaluated with a calibration curve. Except that, the analysis of subgroup (1 LNs and 2–3 LNs) from less regional LNs group was performed. When P value less than 0.05, it means significant. In addition, STROBE and TRIPOD guidelines are performed in the observational study to consult for prediction model.
Results
Patient and tumor characteristics
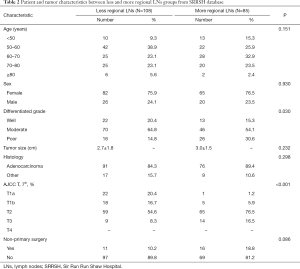
A total of 1,669 coming from SEER were summered in Table 1. There were some differences in two groups, such as patients undergoing lymphadenectomy with more regional LNs group tended to be younger, had lower histological differentiated grade, smaller tumor size, higher T-stages and percent of non-primary. Of these, after propensity score weighting applied to balance covariates in two groups, all covariates were balanced and no longer had statistically significant difference. In addition, the characteristics of 193 patients from our hospital were shown in Table 2.

Full table
Full table
Independent factors and two nomograms
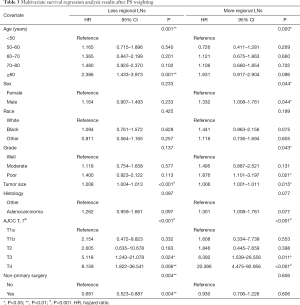
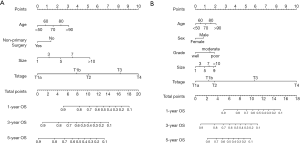
The multivariate survival regression analysis was performed. There were four statistically significant factors for the group of less regional LNs removed, including age (P=0.001), tumor size (P<0.001), T-stages (P<0.001) and receipt of non-primary surgery (P=0.004), which were listed in Table 3. At the meantime, five factors for that of more regional LNs, consisting of age (P=0.020), sex (P=0.044), grade (P=0.043), tumor size (P=0.015) and T-stages (P<0.001), was identified and summered in Table 3. Two nomograms were built on basis of each independent factor. In order to compare the performance of survival models, the lognormal model had the lowest Akaike’s Information Criterion of 9032, indicating a better overall fit than the Cox proportional hazards models (9546). According to the coefficients from this model, two nomograms (Figure 1A,B) were constructed to estimate the survival benefit for lymphadenectomy with less and more regional LNs, respectively. To use the nomogram, first draw a vertical line up to the top point row to assign points for each variable. Then, add up the total points and drop a vertical line from the total point row to obtain the 1-year OS, 3-year OS, and 5-year OS.
Full table
Performance of nomogram
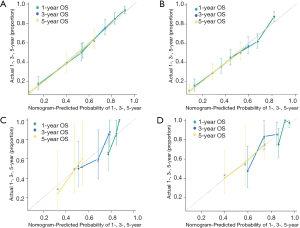
Model performance was internally and externally validated for discrimination and calibration. Discrimination, as measured by the bootstrap corrected C-index, was 0.754 and 0.7103 in internal validation and 0.710 and 0.687 in external validation for more and less regional LNs, respectively. Both of internal calibration curves (Figure 2A,B) and the external calibration curves (Figure 2C,D) showed good agreement between predicted and observed outcomes in the 1-year OS, 3-year OS, and 5-year OS respectively.
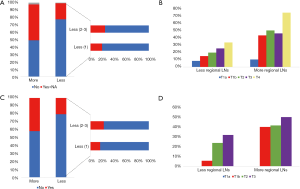
Difference on the status of LNs and each T-stages
Lymphadenectomy with more regional LNs showed a higher percent of positive LNs (P<0.001), according to the data from SEER (Figure 3A). what’s more, the percentage of positive LNs increased with higher T-staged in the less regional LNs group, while that of more regional LNs remained steady (Figure 3B). The same result based on hospital data were shown in Figure 3C,D.
Discussion
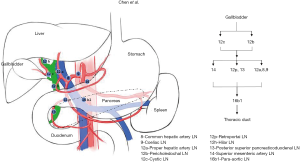
GBC is one of the most common and aggressive biliary tract malignancy (2). Because early GBCs are not always with specific symptoms, and the majority (50–70%) of them are detected as incidental findings after cholecystectomy performed for other indications (12-14). Although the incidental gallbladder cancer (IGBC) is the most common form of GBC diagnosed today (15), many patients present with lymphatic metastases involvement (5). LN status is referred as one of the strongest prognostic factors (6). The early LNs metastasis is a characteristic of GBC (5). Fahim et al. (16) reported that the collecting trunks from the lymphatic plexuses in the medial and lateral wall of the gallbladder terminate in the cystic and peri-choledochal LNs and follow one of three pathways (cholecysto-mesenteric pathways, cholecysto-retropancreatic pathways and cholecysto-coeliac pathways) to converge at the para-aortic LNs between left renal vein and inferior mesenteric artery (Figures S1,S2,S3). Patients with LN metastasis will have a shorter survival time and 30–40% increased risk of death, comparing to patients without LNs metastasis (17,18). However, LNs status may be inaccurate without extensive lymphadenectomy. In our study, we showed that more regional LNs removed have a higher rate of positive LNs, and a more stable accuracy in patients with different T-stage. Some studies also showed same results that it presented with LN metastases in a high proportion of patients, up to 60–80% of T3–4 tumors (7). Although the extensive lymphadenectomy was beneficial for accurately evaluating the nodal basin (19), the operative difficulty and risk was higher. Due to difficulty for surgeons to obtain the LN status directly during surgery, it is very necessary to assist clinicians in decision-making.
Cancer prediction model has been increasingly popular and important in personalized medicine (20), in which clinicians optimize the patient’s therapeutic recommendations according to their specific and individual information. Recently, cancer prediction model have been used in various cancers, such as lung (21-23), breast (24-26), pancreas (27,28) and prostate cancer (29-31). Cancer prediction model usually consists of many observed covariates. Bai et al. (32) constructed a GBC prediction model based on covariates such as jaundice, CA19-9 and T stage to predict OS after GBC resection. However, they merged stage 0 to IIIA into one category, which made the model not accurate and specific. According to SEER database, Zhang et al. (33) developed a nomogram to predict prognosis in patients of GBC (M0) after surgical resection. Although they found that receipt of LN dissection was a significant variable, they didn’t divide LN removal to lymphadenectomy with less regional LNs and lymphadenectomy with more regional LNs, and therefore, some biased existed in this research.
In the present study, we divided patients into less and more regional LNs removal group, meanwhile, we utilized propensity score methods which were usually used to reduce the impact of treatment selection bias, especially for non-random trails (34), to optimize the allocation of data from SEER database, and compared lognormal and Cox proportional hazards model, before we build a final survival model. Although lognormal model is not as popular as Cox proportional hazards model, it has a long history of usage in cancer survival (35) and has been shown to be a more appropriate survival model in some cancers, such as breast cancer (36), lung cancer (37), extrahepatic cholangiocarcinoma (38). Besides, Wang et al. (35) in current study indicated that the lognormal model also demonstrated a good fit for GBC. In this paper, we chose the accelerated failure time parametric model (lognormal), because its Akaike’s Information Criterion was lower than that of semiparametric model (Cox proportional hazards).
Some factors, such as age, size and T-stage, play an important role in OS between less and more regional LNs removed group, while there were some different factors, including non-primary surgery for less regional LNs group, sex and grade for more regional LNs group. Age has a great influence on survival time in the present study as expected. Generally, the elder patients possess a poorer tolerance of stress and a damaged compensatory mechanism, and higher T-stage usually showed more aggressive of the biological behavior of malignant tumors. We found the younger and/ or lower T-stage patients with GBC, the better OS, which was similar to previous studies. Interestingly, we found non-primary surgery or IGBC also a significant factor in the less regional LNs. Patients who underwent non-primary surgery usually showed lower stage, and less regional LNs removed might be enough. The other factor in more regional LNs was no difference with previous studies.
There are some limitations that need to be considered in the present study. Firstly, although the largest series of GBC cases are available form SEER, some of the known survival predictors are nearly all missing in the SEER data, such as margin status, chemoradiotherapy. Its accuracy may be affected, but this nomogram based on information which clinician can obtain before and during surgery. In addition, we used propensity score methods to reduce the impact of treatment selection bias. Therefore, postoperative treatment, such as chemoradiotherapy, immunotherapy and so on, may have little influence on the accuracy of this nomogram. Secondly, this is a retrospective study. The performance of this nomogram shows good in our hospital data, but whether it is suitable for other centers need more data to be improved and testified. Therefore, in the future, we hope get a large external data to optimize this nomogram. Finally, the details of regional LNs are missing, which increases difficulty to study which regional LNs or how much number of regional LNs are recommended to be removed. If possible, our future study will focus on this point.
In summary, we present a novel prediction model that can estimate individual survival benefit of lymphadenectomy with more and/or less regional LNs for resected GBC patients. It can be regarded as a tool to help clinician estimate which people need more regional LNs removed during surgical resection of GBC.
Acknowledgments
Thanks to Yun Cai help us revise and improve this paper.
Funding: This work was supported by Key Research and Development Plan Projects of Zhejiang Province (No. 2017C01018).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Sir Run Run Shaw Hospital Ethics Committee.
References
- Yee K, Sheppard BC, Domreis J, et al. Cancers of the gallbladder and biliary ducts. Oncology (Williston Park) 2002;16:939-46, 949; discussion 949-50, 952-3, 956-7.
- Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167-76. [Crossref] [PubMed]
- Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009;20:146-59. [Crossref] [PubMed]
- Ferretti S, Gafa L. Upper gastrointestinal tract cancers: oesophagus, stomach, liver, gallbladder and biliary ducts, pancreas. Epidemiol Prev 2004;28:34-42. [PubMed]
- Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989-1995. Cancer 1998;83:2618-28. [Crossref] [PubMed]
- Tsukada K, Kurosaki I, Uchida K, et al. Lymph node spread from carcinoma of the gallbladder. Cancer 1997;80:661-7. [Crossref] [PubMed]
- Fong Y, Wagman L, Gonen M, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg 2006;243:767-71; discussion 771-4. [Crossref] [PubMed]
- Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am 2007;16:221-32. [Crossref] [PubMed]
- Kondo S, Takada T, Miyazaki M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg 2008;15:41-54. [Crossref] [PubMed]
- Søreide K, Harrison EM, Wigmore SJ. Research gaps and unanswered questions in gallbladder cancer. HPB (Oxford) 2018;20:685-6. [Crossref] [PubMed]
- Birnbaum DJ, Vigano L, Ferrero A, et al. Locally advanced gallbladder cancer: which patients benefit from resection? Eur J Surg Oncol 2014;40:1008-15. [Crossref] [PubMed]
- Butte JM, Matsuo K, Gonen M, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg 2011;212:50-61. [Crossref] [PubMed]
- Ethun CG, Le N, Lopez-Aguiar AG, et al. Pathologic and Prognostic Implications of Incidental versus Nonincidental Gallbladder Cancer: A 10-Institution Study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg 2017;83:679-86. [PubMed]
- Søreide K, Guest RV, Harrison EM, et al. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg 2019;106:32-45. [Crossref] [PubMed]
- Pitt SC, Jin LX, Hall BL, et al. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg 2014;260:128-33. [Crossref] [PubMed]
- Fahim RB, Mc DJ, Richards JC, et al. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg 1962;156:114-24. [Crossref] [PubMed]
- Liu GJ, Li XH, Chen YX, et al. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J Gastroenterol 2013;19:5150-8. [Crossref] [PubMed]
- Scheingraber S, Justinger C, Stremovskaia T, et al. The standardized surgical approach improves outcome of gallbladder cancer. World J Surg Oncol 2007;5:55. [Crossref] [PubMed]
- Ito H, Ito K, D'Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011;254:320-5. [Crossref] [PubMed]
- Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst 2005;97:715-23. [Crossref] [PubMed]
- Desseroit MC, Visvikis D, Tixier F, et al. Development of a nomogram combining clinical staging with (18)F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur J Nucl Med Mol Imaging 2016;43:1477-85. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Zeng Q, Xue N, Dai D, et al. A Nomogram based on Inflammatory Factors C-Reactive Protein and Fibrinogen to Predict the Prognostic Value in Patients with Resected Non-Small Cell Lung Cancer. J Cancer 2017;8:744-53. [Crossref] [PubMed]
- Dihge L, Bendahl PO, Ryden L. Nomograms for preoperative prediction of axillary nodal status in breast cancer. Br J Surg 2017;104:1494-505. [Crossref] [PubMed]
- Dingemans SA, de Rooij PD, van der Vuurst de Vries RM, et al. Validation of Six Nomograms for Predicting Non-sentinel Lymph Node Metastases in a Dutch Breast Cancer Population. Ann Surg Oncol 2016;23:477-81. [Crossref] [PubMed]
- Tsoutsou PG, Jeanneret Sozzi W, Matzinger O, et al. Nomograms predicting locoregional recurrence in the subtype era of breast cancer. J Clin Oncol 2013;31:647-8. [Crossref] [PubMed]
- Hijioka S, Shimizu Y, Mizuno N, et al. Can long-term follow-up strategies be determined using a nomogram-based prediction model of malignancy among intraductal papillary mucinous neoplasms of the pancreas? Pancreas 2014;43:367-72. [Crossref] [PubMed]
- Jang JY, Park T, Lee S, et al. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients With Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg 2017;266:1062-8. [Crossref] [PubMed]
- Brockman JA, Alanee S, Vickers AJ, et al. Nomogram Predicting Prostate Cancer-specific Mortality for Men with Biochemical Recurrence After Radical Prostatectomy. Eur Urol 2015;67:1160-7. [Crossref] [PubMed]
- Hirasawa Y, Nakashima J, Sugihara T, et al. Development of a Nomogram for Predicting Severe Neutropenia Associated With Docetaxel-Based Chemotherapy in Patients With Castration-Resistant Prostate Cancer. Clin Genitourin Cancer 2017;15:176-81. [Crossref] [PubMed]
- Lughezzani G, Lazzeri M, Haese A, et al. Multicenter European external validation of a prostate health index-based nomogram for predicting prostate cancer at extended biopsy. Eur Urol 2014;66:906-12. [Crossref] [PubMed]
- Bai Y, Liu ZS, Xiong JP, et al. Nomogram to predict overall survival after gallbladder cancer resection in China. World J Gastroenterol 2018;24:5167-78. [Crossref] [PubMed]
- Zhang W, Hong HJ, Chen YL. Establishment of a Gallbladder Cancer-Specific Survival Model to Predict Prognosis in Non-metastatic Gallbladder Cancer Patients After Surgical Resection. Dig Dis Sci 2018;63:2251-8. [Crossref] [PubMed]
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. [Crossref] [PubMed]
- Chapman JA, Lickley HL, Trudeau ME, et al. Ascertaining prognosis for breast cancer in node-negative patients with innovative survival analysis. Breast J 2006;12:37-47. [Crossref] [PubMed]
- Tai P, Chapman JA, Yu E, et al. Disease-specific survival for limited-stage small-cell lung cancer affected by statistical method of assessment. BMC Cancer 2007;7:31. [Crossref] [PubMed]
- Fuller CD, Wang SJ, Choi M, et al. Multimodality therapy for locoregional extrahepatic cholangiocarcinoma: a population-based analysis. Cancer 2009;115:5175-83. [Crossref] [PubMed]