
Bilateral proficiency over time leads to reduced donor morbidity in living donor hepatectomy
Introduction
From its inception in 1989, living donor liver transplantation (LDLT) has revolutionized the field, driving advances in surgical technique and saving countless lives (1-3). While continued innovations in LDLT over three decades have led to recipient outcomes comparable to deceased donor liver transplantation, there is still room for improvement in donor morbidity (4,5). This is especially true in adult LDLT, where risk of postoperative liver failure in the donor is greater because of the need to ensure adequate graft size for sick recipients (1,6). Recent publications have found overall donor complication rates to range from 8.4–49.5% (5,7-9). Of these, biliary complications (BCs) have the potential for the most long-term cost and morbidity and range from 1.8–19.1%, with the latest meta-analysis quoting the incidence at around 6.6% (10-12).
A recent multi-center review by Rössler et al. emphasized that center experience and graft side are important factors in reducing the risk of donor morbidity after hepatectomy (5). They reported that left graft donation is associated with lower complication rates and severity compared to right graft donation. These results were consistent with multiple previous comparisons of right vs. left hepatectomy (6,13-15). Given these findings, many centers have gravitated towards using left grafts in appropriate cases, with the goal of reducing donor risk (8,16,17). However, save for a few centers in Japan prioritizing left grafts (18,19), right-sided donor hepatectomy still predominates in adult LDLT (aLDLT)—in North America, only 5% of adult living donor grafts are left grafts, and a recent multicenter study compiling data from 5,202 patients over eleven years only contained 19% left lobe grafts (1,5,14). Limited left graft experience at individual centers precludes in-depth evaluation of learning curve outcomes and graft-side comparisons by complication type.
Our center started performing left donor hepatectomy for aLDLT in February 2001. Since then, the proportion of left lobe donations has increased to more than one-third of our procedures. Over many years of experience, facility with either right or left donor hepatectomy has become a hallmark of our practice. After careful pre-operative evaluation, for donors with adequate volumetry and suitable anatomy for either side, we can decide intraoperatively which side to take after direct visualization of the donor liver, its vasculature and intraoperative cholangiography (IOC). We aimed to evaluate our extensive experience with aLDLT, focusing on whether increasing left lobe cases and bilateral proficiency with donor hepatectomy can optimize operative characteristics and reduce donor complications.
Methods
Patient selection
All 834 consecutive adult-to-adult LDLT (≥18 years) donors operated on at Kaohsiung Chang Gung Memorial Hospital, Taiwan between January 2004 and December 2014 were enrolled into this retrospective study. The study population was divided into two eras (Era 1 2004–2010, Era 2 2011–2014), based on frequency of left hepatectomy. Stratified analysis of these two time-periods was performed, focusing on graft-side comparisons. Data collected included relevant demographic variables such as donor age, sex, height, weight, smoking status, and calculated liver volume (CLV) based on computed tomography scan (20). Graft weight was measured intraoperatively after back table flush and was used to calculate remnant volume percentage utilizing the following formula: (CLV − graft weight)/CLV. Pertinent intraoperative findings recorded included blood loss, graft weight, operative time, and hepatectomy side. All cases analyzed were anatomic right or left hepatectomies, with left lateral segment grafts, extended left lateral segment grafts or right posterior segment grafts excluded. In general, we include the middle hepatic vein (MHV) with left- but not right-sided donor grafts. The only exceptions in this cohort were 34/613 right lobe grafts which included the MHV and 13/221 left lobes sans the MHV.
Complications
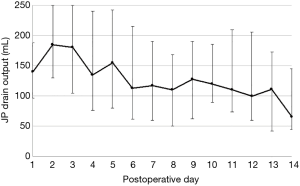
Donor complication data focusing on all inpatient and overall complications within 6 months was collected and graded according to the Clavien-Dindo grading system to create a comprehensive complication index score (21,22). Complications graded ≥ IIIa were deemed major complications. Ascites was defined as abdominal drain output >250 mL on or after postoperative day (POD) 5 and/or prolonged drain output >150 mL on POD10 or later. These outlier thresholds were chosen after analyzing a random sample (n=116) of our patient population and defining the interquartile range (see Figure 1). Drain outputs were measured utilizing a 7-mm Flat Jackson-Pratt drain to bulb suction placed in all donors adjacent to the liver fossa. To our knowledge, there has been no previous definition of ascites specifically for living donor hepatectomies; most published definitions of post-hepatectomy ascites come from the cancer patient population (23,24), which may not be representative of our study population. Thus, we chose to define this complication based on our own data from healthy donors. BCs included leaks (graded per ISGLS guidelines), and strictures (25). Complications were categorized into individual types, which included ascites, BCs, intra-abdominal bleeds and collections, urinary tract infections, ulcers or gastritis, and ileus. Post-hepatectomy liver failure (PHLF) was defined according to the International Study Group of Liver Surgery definition, with cutoffs of INR ≥1.3 & bilirubin ≥1.2 mg/dL on or after POD 5 (26). This study was approved by the institutional review board of Kaohsiung Chang Gung Memorial Hospital (IRB No.104-9281B).
Pre-op workup and graft selection
A detailed description of the pre-operative donor workup at our institution has been published previously (27). In general, we aim for a graft that is at least 35% of the recipient’s standard liver volume (SLV), and leaves a remnant liver volume of >30% in the donor. Even if the left lobe meets these volumetric criteria, we have strict selection criteria based on vascular anatomy. Left hepatectomy with MHV is excluded if the donor has a prominent MHV with small caliber right hepatic outflow. Proper venous outflow for both donor and recipient is a critical factor in our graft side decision-making to ensure optimal recovery (28). Although most of our hepatectomy side decisions are made preoperatively, in some cases where either side is volumetrically suitable for the recipient, we reserve the final decision until intraoperative visualization of the liver, its vasculature and IOC. Vascular or biliary anomalies are rarely the sole reason for donor exclusion (29). Donors with left grafts too small to meet the volume criteria but whose right lobe graft would leave too little remnant volume are declined, unless the vascular and biliary anatomy are suitable for right posterior segment graft procurement.
Technique
All donor operations were helmed by the same primary surgeon (CLC). Our open donor hepatectomy technique has been previously described (30). The salient points that our institution ascribes to are: routine IOC use, meticulous preservation of the vascular supply to the biliary tree with the complete hilar plate encircling technique (31), oversewing the graft and remnant hilar plate and any visible bile duct orifices with 6-0 Prolene after transection, and minimizing overall blood loss. During the parenchymal transection we do not use the Pringle maneuver. Instead, central venous pressure (CVP) is maintained between 5–10 mmHg with low volume maintenance fluids (2–4 mL/kg/hr) and diuretics as needed, and the liver tissue is carefully transected using a combination of clamp-facture, CUSA and electrocautery. Vascular and biliary structures are generally divided between ligatures and not stapled. Before encircling the hilar plate, small portal branches to the S4 and caudate lobe are ligated and divided. IOC visualization of the biliary tree and planned line of transection is confirmed prior to sharply dividing the hilar plate. The MHV is included with the left lobe graft, and segment 5 & 8 vein stumps are oversewn in the remnant. At the end of the case, the donor remnant liver is routinely biopsied, and an IOC and Doppler ultrasound are performed at the end of the case to ensure that the remnant donor anatomy is intact. An intra-peritoneal Jackson-Pratt (JP) drain is placed at the end of each case adjacent to the resection bed.
Postoperative care
Donors are monitored in a surgical intensive care setting postoperatively, with standard perioperative antibiotics, resuscitation goals of urine output >0.5 mL/kg/hr and CVP 8–12 mmHg, and venous thromboembolism prophylaxis via pneumatic compression and early mobilization. JP drain outputs are recorded daily, and are removed when output is less than 100 mL per 24 hours without evidence of active bleeding or bile leakage. Post living donor hepatectomy massive ascites is exceedingly rare.
Statistics
Data collection and analysis were performed using SPSS version 21 (SPSS, Inc., Chicago, IL, USA) and STATA version 14 (StataCorp LP, College Station, TX, USA). Continuous variables were expressed as means with standard deviation (SD) except where noted. Group means were compared using the Student’s t-test and medians compared via the Mann-Whitney U test. Categorical data was expressed as percentage and mean, and group comparisons were performed using Fisher’s exact test. For multiple regression analyses, univariate logistic regression was performed first. Marginal predictors with P value <0.10 were subsequently placed into a multivariate model. A P value <0.05 was considered statistically significant for all analyses.
Results
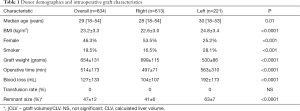
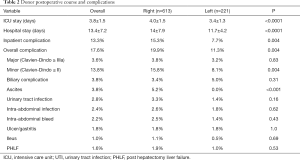
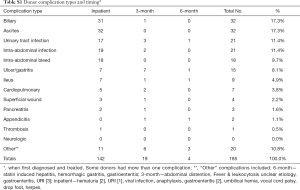
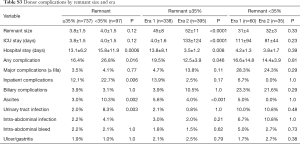
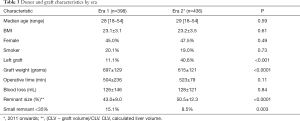
In total, 834 donors (46.3% female) with a median age of 29 (range, 18–54) years underwent formal left or right donor hepatectomy for adult recipients at our center between January 2004 and December 2014. Table 1 displays patient characteristics and operative parameters for the overall study population and by hepatectomy side. In total, 221 (26.5%) of the donors donated left grafts. Compared to right-lobe donors, left-lobe donors tended to be male, with higher BMI’s, and were more likely to be smokers. As expected, left lobe grafts were smaller (530±86 g left vs. 699±115 g right, P<0.0001), leaving a larger remnant size in donors (63%±7% left vs. 41%±6% right, P<0.0001). Left hepatectomy cases had significantly longer operative time (563±310 min left vs. 497±71 min right, P<0.0001) and blood loss (192±173 mL left vs. 104±107 mL right, P<0.0001). The overall 6-month complication rate was 17.6% (147/834), with 3.6% (30/147) of donors experiencing severe complications Clavien-Dindo grade 3 or higher (Table 2). The most common complications were BCs and ascites, each of which accounted for 17.3% of all complications. The next most frequent complications were UTI, intra-abdominal infection, intra-abdominal bleed, and ulcer/gastritis (Table S1). Donors who experienced major complications were most likely to have biliary [14], intra-abdominal bleed [5] or intra-abdominal infections (2 non-biliary, 5 biliary), pleural effusion [1], duodenal ulcer [6] (Table S2). Two donors had grade IV complications: one due to anaphylactic shock, and one thrombotic event related to IVC thrombus causing bilateral lower extremity edema, which was managed successfully by nonoperative approach with Coumadin. There was no donor mortality in our entire series of LDLT.
Full table
Full table
Full table

Full table
Complication rates and characteristics differ by hepatectomy side
Right lobe donors had significantly more overall (19.9% R vs. 11.3% L, P=0.004) and inpatient (15.3% R vs. 7.7% L, P=0.004) complications than left lobe donors and had longer intensive care unit (ICU) and inpatient hospital stays. Postoperative ascites was confined exclusively to the right-lobe donor population (5.2% R vs. 0.0% L, P<0.001). Otherwise there were no significant differences in severe complications, biliary, infectious, abdominal bleed, ulcer/gastritis complications, or PHLF between right and left donor hepatectomy. These contrasts persisted on subgroup analysis within the population of patients with donor remnants >35% (data not shown), demonstrating that even with sufficient remnant volume, right graft donors still had higher morbidity risk. In fact, of the twelve cases of PHLF, ten (83.3%) were from right lobe donors. Of these, half were ISGLS grade A (without complication and not requiring change in postoperative management).
Increased left-graft case volume lowers complication rates and severity
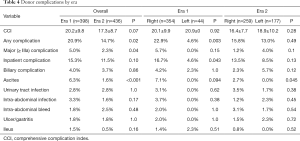
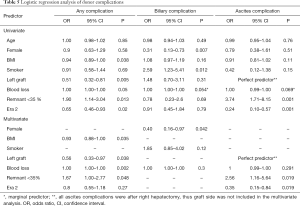
The first left adult-to-adult LDLT performed at our center was in February 2001. Subsequently, our experience with left grafts greatly increased starting in 2011 (from ≤15 left lobes a year to >30 and up to 57 per year, Table S3). Dividing all cases into two eras, we investigated the impact of increasing left graft donation on donor outcomes. The proportion of left donor hepatectomies more than tripled in Era 2 (from 11.1% to 40.6% of all donor grafts, P<0.001), leading to expected decreases in mean graft weight and increases in donor remnant size (Table 3). All other donor demographics stayed similar between eras. Importantly, overall and major complications decreased significantly in Era 2 (overall: 14.7% Era 2 vs. 20.9% Era 1, P=0.02; major: 2.3% Era 2 vs. 5.0% Era 1; Table 4). On analysis of specific complication types, a significant decrease in ascites incidence was seen in Era 2 (1.6% Era 2 vs. 6.3% Era 1, P<0.001), but other complication rates remained similar between eras. On univariate analysis, donor BMI, higher blood loss, and remnant size <35% were significant predictors of having a complication, while left graft donation and operation during Era 2 were protective. On multivariate analysis, all these predictors except for Era stayed significant (Table 5).
Full table
Full table
Full table
Full table
Right vs. left hepatectomy complications equilibrate with experience
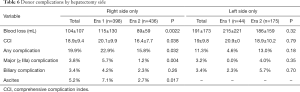
Examining the right lobe donor population only, these donors experienced significantly less blood loss (89±59 Era 2 vs. 115±130 Era 1, P=0.0022; Table 6) and fewer complications (15.8% Era 2 vs. 22.9% Era 1, P=0.032) in Era 2, with lower severity (CCI 16.4±7.7 Era 2 vs. 20.1±9.9 Era 1, P=0.038; major complications 1.2% Era 2 vs. 5.7% Era 1, P=0.004) and ascites complications (2.7% Era 2 vs. 7.1% Era 1, P=0.017; Table 6). Interestingly, in Era 2, the right hepatectomy complication rate became comparable to that of left hepatectomy (15.8% R vs. 13.0% L, P=0.49), which contrasted with the significant difference between sides seen in Era 1 (22.9% R vs. 4.6% L, P=0.003). Meanwhile, left sided differences in operative characteristics persisted in Era 2, with longer operative time and increased blood loss compared to right lobes (data not shown).
Full table
BC severity decreases with experience
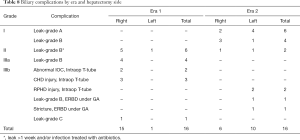
The overall donor BC rate was 3.8% (32/834). Of these, most (25/32, 78.1%) were related to leaks. Although the BC rate stayed similar in both eras (4.0% Era 2 vs. 3.7% Era 1, P=0.86, Table 4), the proportion of severe BCs (≥ Clavien-Dindo Grade 3) was more than halved in Era 2 compared to Era 1 (25.0% Era 2 vs. 62.5% Era 1, P=0.073; Table 7). Table 8 details the types, severity, and applicable interventions for these BCs. On univariate analysis, the only significant predictors of donor BCs were positive smoking status (OR =2.59, P=0.012, Table 5), and female gender, which was protective (OR =0.31, P=0.007). On multivariate analysis only gender remained a statistically significant predictor of BCs.

Full table
Full table
Stable recipient outcomes over time
With high utilization of left-grafts, there is concern that recipient outcomes may be compromised. We compared our graft and patient survival rates by hepatectomy side and found no statistically significant differences (Table S4). Briefly, 12-month graft and patient survival rates were comparable: 95.9% in right grafts and 93.4% in left grafts.

Full table
Discussion
LDLT is one of the most striking examples of altruism in medicine. Healthy donors take on a life-risking procedure to save their loved ones, placing the onus on the surgeon to minimize morbidity, regardless of how much the donor is willing to “sacrifice”. This principle has motivated continual improvements in donor hepatectomy technique as well as choice of graft side (14,32). While most recent donor outcome publications have centered on right donor hepatectomy (9,33,34), development of left donor hepatectomy has gained traction because of its potential to reduce donor morbidity as well as concurrent advances in recipient management that allow smaller grafts to be safely transplanted (14,16,35,36). In this study, we examined a large cohort of patients from a single center, operated on by the same primary surgeon from 2004 to 2014. With a relatively constant care team, methodical technical improvements over the decade, and increasing left hepatectomy volumes, our center was an ideal environment to explore the effect of center experience on donor outcomes after hepatectomy.
Consistent with previous comparisons of right vs. left donor hepatectomy, we initially found that right lobe donors tended to have higher complication rates, more severe complications, and worse morbidity than their left-donating counterparts (5,14,15,37). This supports our initial hypothesis that increasing volumes of left lobe donation would lead to an overall decrease in donor morbidity, as demonstrated by the reduction in overall and major complications during Era 2 compared to Era 1. However, on closer examination, we found that the difference in complication rates between right and left lobes was only present during the first era, and was not detected during the second era. It is possible that a difference could have emerged with an even larger sample size, but we suspected that it was not just the presence of more left lobes that brought the complication rate down. Upon analyzing the right lobe donor population, we found a clear (and statistically significant) reduction in right hepatectomy complications that contributed to equilibration of donor complication risk between sides. This suggests that as more centers around the world pursue left graft donation (and left hepatectomy complications concomitantly increase with initial inexperience and higher volumes), we may find that optimizing donor safety will depend less on graft side, and more on other parameters, such as minimizing intraoperative blood loss and remnant steatosis (38,39).
Because complication incidence and severity decreased in Era 2, this suggests that improvements in surgical technique and patient care led to better donor outcomes. Upon closer examination of specific complication types, we found that major contributors to donor morbidity were BCs, intra-abdominal bleeds, delayed ulcer disease, and ascites. Of these, only ascites incidence decreased significantly during the second era, accounting for the lower complication rate. Ascites is a low-grade complication that rarely required intervention, but its presence was a marker for prolonged hospital and ICU stay, and can lead to increased costs from delayed mobilization, prolonged drain placement, and higher risk for infection and intra-abdominal adhesions. We suspect that the decrease in ascites was spurred not only by increased utilization of left lobes, but better perioperative management of patient fluid status.
Although our BC rate did not decrease significantly in the second era compared to the first, the marked reduction in degree of severity implies that donor safety improved with center experience. Since BCs can lead to long-term donor morbidity, our focus on minimizing them cannot be overemphasized. To our knowledge, our overall living donor BC rate of 3.8% is the lowest in the literature for studies analyzing pure right or left donor hepatectomy for adult LDLT without the inclusion of left lateral segment or other graft types (10). Although some Japanese centers have reported that the risk of BCs is higher in right hepatectomy (6,7), in our study, we did not find this to be the case. Recent reports from high-volume LDLT centers have reported exceptionally low donor BC rates, including a large single-center study from Shin et al. (analyzing predominantly right hepatectomy) with a 1.8% BC rate and a study from Taketomi et al. reporting a BC rate of 4.1% (11,12). Their analyses had conflicting conclusions on whether experience influences complication rates, with Shin et al. finding no improvement in overall or BC rates over time, while Taketomi et al. found a significant reduction in BCs (from 6.4% to 1.8%) during the latter half of their study period (6,11). The latter group highlighted specific techniques leading to reduction of BCs that are also practiced at our center: (I) meticulous dissection with complete hilar encircling technique to maximize the biliary blood supply (31); (II) routine IOC; and (III) oversewing the hilar plate after transection. The main difference between their method and ours is that we do not utilize the Pringle Maneuver. We believe that maintaining a low positive CVP while avoiding disruptions to the parenchymal blood supply allows easier control of bleeding, and results in better outcomes for both the donor and the recipient.
While shared advances in surgical technique have spurred improvements in donor safety at centers worldwide, we believe that the evolution of decision-making in LDLT is a critical piece of the puzzle that is also greatly influenced by center experience. One unique feature of our center is our ability to make intra-operative decisions on which liver lobe to take during donor hepatectomy. In our early experience, when familiarity was mainly with right hepatectomy, aberrancies such as inaccurate volumetry or unexpected anatomy sometimes led to aborted donor surgeries. While these occasions were rare, they motivated us to continually improve our expertise such that our imaging techniques and pre-operative workup are now exquisitely fine-tuned to optimize donor selection. One unanticipated benefit from these innovations is that we have become so comfortable with left or right donor hepatectomy that we can wait until direct intraoperative visualization of the donor liver to finalize graft choice. Pre-operatively, all cases are reviewed and graft side is chosen with input from radiologists and the surgical team. In most cases, pre-operative plans are upheld, but there are rare instances where we change sides in the interest of donor safety. These decisions are often based on direct visualization of liver lobe size, and confirmation of aberrant anatomy. This flexibility is significant, because at centers that are less familiar with left hepatectomy, the preference for right hepatectomy may result in unnecessarily small donor remnants (increasing the risk for donor morbidity), or a higher rate of aborted surgeries, both of which may confer higher costs and distress for the patients and their families (32).
Moving forward, upcoming challenges to donor safety include the rise of fatty liver disease, as well as changing indications for transplant. As endemic hepatitis B and C incidence decreases, HCC will be supplanted by other diseases such as decompensated alcoholic cirrhosis or NAFLD, which may require different approaches (2,40,41). In the face of these changes, we believe that continued technical innovations and facility with both right and left hepatectomy will optimize donor safety, providing the tools to minimize donor risk and morbidity.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of Kaohsiung Chang Gung Memorial Hospital (IRB No.104-9281B).
References
- Kim PTW, Testa G. Living donor liver transplantation in the USA. Hepatobiliary Surg Nutr 2016;5:133-40. [PubMed]
- Pillai VG, Chen CL. Living donor liver transplantation in Taiwan-challenges beyond surgery. Hepatobiliary Surg Nutr 2016;5:145-50. [PubMed]
- Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015;15:17-38. [Crossref] [PubMed]
- Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant 2012;12:1208-17. [Crossref] [PubMed]
- Rössler F, Sapisochin G, Song G, et al. Defining Benchmarks for Major Liver Surgery: A multicenter Analysis of 5202 Living Liver Donors. Ann Surg 2016;264:492-500. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Nakagawara H, et al. Revisiting the Safety of Living Liver Donors by Reassessing 441 Donor Hepatectomies: Is a Larger Hepatectomy Complication-Prone?: Revising the Safety of Living Liver Donors. Am J Transplant 2014;14:367-74. [Crossref] [PubMed]
- Hashikura Y, Ichida T, Umeshita K, et al. Donor complications associated with living donor liver transplantation in Japan. Transplantation 2009;88:110-4. [Crossref] [PubMed]
- Halazun KJ, Przybyszewski EM, Griesemer AD, et al. Leaning to the Left: Increasing the Donor Pool by Using the Left Lobe, Outcomes of the Largest Single-center North American Experience of Left Lobe Adult-to-adult Living Donor Liver Transplantation. Ann Surg 2016;264:448-56. [Crossref] [PubMed]
- Facciuto M, Contreras-Saldivar A, Singh MK, et al. Right hepatectomy for living donation: role of remnant liver volume in predicting hepatic dysfunction and complications. Surgery 2013;153:619-26. [Crossref] [PubMed]
- Braun HJ, Ascher NL, Roll GR, et al. Biliary complications following living donor hepatectomy. Transplant Rev (Orlando) 2016;30:247-52. [Crossref] [PubMed]
- Taketomi A, Morita K, Toshima T, et al. Living donor hepatectomies with procedures to prevent biliary complications. J Am Coll Surg 2010;211:456-64. [Crossref] [PubMed]
- Shin M, Song S, Kim JM, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation 2012;93:942-8. [Crossref] [PubMed]
- Taketomi A, Kayashima H, Soejima Y, et al. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation 2009;87:445-50. [Crossref] [PubMed]
- Roll GR, Parekh JR, Parker WF, et al. Left hepatectomy versus right hepatectomy for living donor liver transplantation: shifting the risk from the donor to the recipient. Liver Transpl 2013;19:472-81. [Crossref] [PubMed]
- Iida T, Ogura Y, Oike F, et al. Surgery-related morbidity in living donors for liver transplantation. Transplantation 2010;89:1276-82. [Crossref] [PubMed]
- Soejima Y, Shirabe K, Taketomi A, et al. Left lobe living donor liver transplantation in adults. Am J Transplant 2012;12:1877-85. [Crossref] [PubMed]
- Braun HJ, Dodge JL, Roll GR, et al. Impact of Graft Selection on Donor and Recipient Outcomes After Living Donor Liver Transplantation. Transplantation 2016;100:1244-50. [Crossref] [PubMed]
- Ikegami T, Yoshizumi T, Sakata K, et al. Left lobe living donor liver transplantation in adults: What is the safety limit?: Left Lobe Living Donor Liver Transplantation in Adults: What Is the Safety Limit? Liver Transpl 2016;22:1666-75. [Crossref] [PubMed]
- Akamatsu N, Kokudo N. Living Liver Donor Selection and Resection at the University of Tokyo Hospital. Transplant Proc 2016;48:998-1002. [Crossref] [PubMed]
- Cheng YF, Huang TL, Chen TY, et al. Liver graft regeneration in right lobe adult living donor liver transplantation. Am J Transplant 2009;9:1382-8. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- Ishizawa T. Risk Factors and Management of Ascites After Liver Resection to Treat Hepatocellular Carcinoma. Arch Surg 2009;144:46. [Crossref] [PubMed]
- Hoekstra LT, Wakkie T, Busch ORC, et al. Predictors of Posthepatectomy Ascites with or without Previous Portal Vein Embolization. Dig Surg 2012;29:468-74. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Chen YS, Cheng YF, De Villa VH, et al. Evaluation of living liver donors. Transplantation 2003;75:S16-19. [Crossref] [PubMed]
- Tsang LLC, Tung YC, Hsu HW, et al. Impact of Graft Type in Living Donor Liver Transplantation: Remnant Liver Regeneration and Outcome in Donors. Transplant Proc 2016;48:1015-7. [Crossref] [PubMed]
- Tsang LLC, Chen CL, Huang TL, et al. Preoperative imaging evaluation of potential living liver donors: reasons for exclusion from donation in adult living donor liver transplantation. Transplant Proc 2008;40:2460-2. [Crossref] [PubMed]
- Chen CL, Chen YS, de Villa VH, et al. Minimal blood loss living donor hepatectomy. Transplantation 2000;69:2580-6. [Crossref] [PubMed]
- Lin TS, Concejero AM, Chen CL, et al. Routine microsurgical biliary reconstruction decreases early anastomotic complications in living donor liver transplantation. Liver Transpl 2009;15:1766-75. [Crossref] [PubMed]
- Pomposelli JJ. Liver remnant volume after living donor liver transplantation: How low should we go? Liver Transpl 2013;19:796-7. Editorial. [Crossref] [PubMed]
- Kim SH, Kim YK, Lee SD, et al. Selection and outcomes of living donors with a remnant volume less than 30% after right hepatectomy. Liver Transpl 2013;19:872-8. [Crossref] [PubMed]
- Guler N, Yaprak O, Gunay Y, et al. Major complications of adult right lobe living liver donors. Hepatobiliary Pancreat Dis Int HBPD INT 2015;14:150-6. [Crossref] [PubMed]
- Kurihara T, Yoshizumi T, Yoshida Y, et al. Graft selection strategy in adult-to-adult living donor liver transplantation: When both hemiliver grafts meet volumetric criteria. Liver Transpl 2016;22:914-22. [Crossref] [PubMed]
- Troisi RI, Berardi G, Tomassini F, et al. Graft inflow modulation in adult-to-adult living donor liver transplantation: A systematic review. Transplant Rev (Orlando) 2017;31:127-35. [Crossref] [PubMed]
- Iwasaki J, Iida T, Mizumoto M, et al. Donor morbidity in right and left hemiliver living donor liver transplantation: the impact of graft selection and surgical innovation on donor safety. Transpl Int 2014;27:1205-13. [Crossref] [PubMed]
- Ibrahim S, Chen CL, Lin CC, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transpl 2006;12:950-7. [Crossref] [PubMed]
- Cheng YF, Yu CY, Ou HY, et al. Section 1. Image Evaluation of Fatty Liver in Living Donor Liver Transplantation. Transplantation 2014;97:S3-6. [Crossref] [PubMed]
- Zezos P. Liver transplantation and non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:15532. [Crossref] [PubMed]
- Ahn CS, Hwang S, Kim KH, et al. Long-term outcome of living donor liver transplantation for patients with alcoholic liver disease. Transplant Proc 2014;46:761-6. [Crossref] [PubMed]


