
Hepatobiliary surgeons meet immunologists: the case of colorectal liver metastases patients
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer-related deaths worldwide (1). Since the liver is the main filter of the venous drainage of the bowel, most patients with CRC develop colorectal liver metastases (CLM). Hepatic resection for CLM combined with systemic therapy has the potential to be curative for patients with CLM, as this therapeutic approach has been associated with 5- and 10-year survival rates up to 50% and 35%, respectively (2). Indeed, fluoropyrimidine-based combinations (FOLFOX and FOLFIRI) with or without biological therapies using anti-vascular endothelial growth factor (VEGF) or anti-epidermal growth factor receptor (EGFR) inhibitors have changed the natural history of CLM (3). This combined approach offers to a significant proportion of patients, who would not have been considered for surgery until a few years ago, the possibility to undergo hepatic resection. However, failure of this contemporary management is not uncommon, and in this case, prolonged survival is unusual (4,5).
In the last years, the field of cancer immunology has caught the attention of surgical and medical oncologists, as immune-based anticancer strategies, including checkpoint inhibitors, have been applied to different clinical scenarios with promising results (6-8). Anti-PD-1 or anti-PD-L1 blocking antibodies have been used in CRC patients (6,9). However, significant results have been shown only in those patients with microsatellite instability (MSI), which represent up to 15% of the whole CRC population (10,11). Moreover, in the context of CRC in particular, a careful evaluation of immune infiltrating cells is emerging as an important prognostic tool to classify cancer patients according to the features of their immune landscape (12,13). Thus, while we are still far from fully understanding the interactions between the immune system and cancer in CLM patients, efforts aimed at clarifying the oncoimmunological dynamics are expected to aid in the introduction of new immune-based therapeutic strategies and improved patient stratification. A major obstacle to a refined definition of the immune contexture of human CLM resides also in the profound heterogeneity of tumor lesions across patients as well as intermetastatic (12). This scenario is also complicated by the frequent neoadjuvant treatment of CLM patients; in fact, both chemotherapy and targeted therapy could potentially affect the type of immune infiltrate. From two real representative cases, in this article we review the immune contexture of CLM patients as recently discovered, illustrating the diversity of immune infiltrate.
Representative case 1
A 52-year-old female was diagnosed with adenocarcinoma of the left colon with synchronous bilateral CLM. The tumor was KRAS wild-type with microsatellite stability (MSS). Since the primary tumor was asymptomatic, she was treated with FOLFOX for 6 courses with radiological partial response in the sigma and in the liver lesions. Then, she underwent multiple liver resections with the removal of 11 histologically proven CLM. She completed 6 courses of FOLFOX. Finally, she underwent left colectomy. Thirteen months after the last surgical operation, she progressed with 3 new CLM. She underwent 4 courses of FOLFIRI with cetuximab with evidence of radiological partial response. She underwent another liver surgery with the removal of the 3 CLM. Thus, she completed the second line of systemic therapy with another 6 courses of FOLFIRI. To date, she is alive and free of disease 33 months from the initial diagnosis.
Figure 1 represents histological sections of the intratumor and peritumor areas of the resected CLM. Immunohistochemical staining was performed as follows: 2 micrometer thick tissue slides from FFPE tumor sections were deparaffinized, and rehydrated. Antigen retrieval was performed using EDTA buffer (0.25 mM, pH 8) at 98 °C for 20 min, followed by block of endogenous peroxidases with 3% hydrogen peroxide (Sigma) and block of nonspecific sites with Background Sniper (Biocare Medical). The sections were then incubated with primary antibodies: CD68 (Dako, KP-1 clone, 1:1,000), CD3 (Thermo Fisher, SP7 clone, 1:100), and NKp46 (Abcam, polyclonal, 1:300), followed by incubation with detection system MACH 1 (Biocare Medical). Sections were then incubated with DAB (Biocare Medical), counterstained with hematoxylin, dehydrated and mounted. As shown, there was a very high density of tumor infiltrating T and NK cells, as shown by the immunohistochemical staining of CD3+ and NKp46+ cells. In the peritumor invasive margin, also a low density of macrophages was observed, as documented by the staining of CD68+ cells.
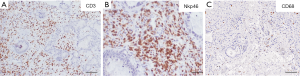
Representative case 2
A 37-year-old male was diagnosed with adenocarcinoma of the right colon with synchronous bilateral CLM. The tumor was KRAS mutated and had MSS. The primary tumor was symptomatic because of bleeding, and the patient was treated with right colectomy. Next, he received systemic therapy with 6 courses of FOLFOX and bevacizumab with evidence of radiological partial response. He was then considered for liver surgery and treated with multiple liver resections to remove 8 CLM. Later on, he progressed on postoperative FOLFOX and bevacizumab because of new CLM and bilateral lung metastases. He then started second line systemic therapy with FOLFIRI and aflibercept and remained stable for 8 months. He was reconsidered for loco-regional therapies (liver and lung resections), but he progressed significantly while on waiting list for liver surgery. He started a third line of systemic therapy with regorafenib and also received trans-arterial radioembolisation of the liver. None of these treatments achieved durable results, and he died 18 months after the initial diagnosis.
Figure 2 shows histological sections of the intratumor and peritumor area of the resected CLM. Immunohistochemical staining was performed as previously described. In this case, as opposed to the previous representative case, there was a low density of tumor-infiltrating T and NK cells as evidenced by the immunohistochemical staining of CD3+ and NKp46+ cells. In the peritumor invasive margin, in contrast, a high density of macrophages was observed (CD68+ cells).
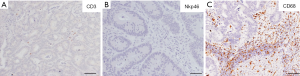
These two case reports clearly depict the heterogeneity of the immune infiltrate in human CLM, in terms of density and type of cells. Notably, the myeloid component (i.e., macrophages) was in sharp contrast with the lymphocytic one, suggesting that these two immune populations could have opposite prognostic values. A comprehensive analysis of the immune contexture capturing both lymphoid and myeloid components would be more informative and possibly disclose important prognostic immune variables.
The immune landscape of CRC liver metastases patients
The immune system includes the innate and the adaptive components. The first component includes the response mediated by NK cells, macrophages and dendritic cells. These cells are the frontline, and they are triggered by bacterial products, endotoxins and pathogenic nucleic acids. The second component includes T and B cells that, once activated, provide the basis for immune-specific protection. Of note, these two components of the immune system work together, and, in fact, there is a significant overlap in the activation process of these cells.
Cancer is a disease characterized by a considerable, but variable, number of genetic alterations and by a loss of normal cellular regulatory processes (14). These genetic alterations result in the expression of molecules that can activate the innate immune system, as well as neoantigens expressed on the surface of cancer cells that can be recognized by T cells. These mechanisms are the theoretical premises for an effective host antitumor immune response. However, unfortunately tumors are able to conceive escape strategies to elude immune control.
The liver is an organ endowed with peculiar immunological properties. Under normal conditions, a human adult liver contains 1010 lymphocytes, the majority of these cells being cytotoxic T and NK cells (14-16). Indeed, elevated local levels of chemokines, such as CCL5, CCL2 and IL-8 promote leucocyte migration and accumulation in the hepatic parenchyma (17,18). A large amount of data available in the literature advocates that the infiltrating leucocytes have a major effect on the clinical outcome of patients with liver metastases (12,16-24). The tissue microenvironment of the metastatic liver is, in fact, characterized by increased levels of inflammatory cytokines and perturbations of chemokine expression. The molecular cross talk among tumor-infiltrating immune cells, local tissue-resident immune cell populations, stromal cells and malignant cells determines the fate of the metastatic disease (25). This increased local inflammation may upset the normal hepatic immune cell repertoire and disrupt tumor immune surveillance (26). Hence, understanding the mechanisms inducing the infiltration of immune cells in the CLM is of paramount importance both for the estimation of the prognosis and for the development of novel and more effective therapies. In general, tumor-infiltrating immune cells have been associated with prolonged survival in patients with colon cancer (12,13,19,27-31). Mlecnik et al. (24) demonstrated a stronger association between T cell infiltration and prognosis in CRC over the traditional TNM staging system. However, most of the patients cannot orchestrate an effective immune response against cancer cells, making the comprehension of this phenomenon very complex. Moreover, the high heterogeneity of the clinical presentation of CLM, which is responsible for the wide array of responsiveness to treatments, could rely on the individual immune response. In a following work, the same group investigated the prognostic relevance of T cell infiltration in CLM and confirmed that response to treatment and prolonged survival of metastatic CRC patients were statistically significantly associated with high densities of immune cells (12). In addition to this, Donadon et al. (32) have recently evaluated the clinical relevance of the immune microenvironment in CLM patients, showing that intratumor T and NK cells were independent prognostic factors favoring overall survival after resection of CLM. Again, these prognostic factors were found to be much more informative than the TNM staging system and than many other traditional clinico-pathological factors that are used in clinical practice to stratify the prognosis of CLM patients.
Figure 3 shows a representative cartoon of a CLM lesion highlighting the abundance of immune cells that may be found in the peritumor area. Each of the cells represents an element potentially contributing to the clinical heterogeneity of CLM patients, and could represent a useful tool for the development of new biomarkers and hence new personalized immunotherapies.

Spatial distribution of immune cells in cancer is highly variable (33). The geometry of the spatial disposition of cancer cells, stromal cells, vessels, normal cells and immune cells is emerging as one of the key factors of the analyses. The density of the multiple types of immune cells infiltrating tumor tissues might be just one of the important features to consider when assessing the relevance of immune cells in cancer, and research in the field should develop a multiplex tool that takes into account the morphology, the functional characterization and the spatial arrangement of immune cells in cancer. Fruitful interactions among different immune cells might also have a prognostic value. For instance, the colocalization of T cells and neutrophils in a specific area in CRC patients was found to be associated with a better outcome (34). The spatial layout is even more important in the case of metastases, since the development of the metastatic niche requires multiple complex events. Celià-Terrassa et al. (35) recently showed how a multi-step process (anchorage, survival, protection and proliferation) is required to build a metastasis-receptive microenvironment. Along these lines, tumor-immune interplay is of paramount importance. The recruitment of immunosuppressive cells is, in fact, one of the first events in the process leading to the formation of the metastatic niche. Alternatively activated macrophages, neutrophils and myeloid-derived suppressor cells have been shown to suppress T cell infiltration and activation, thus promoting metastases (36,37).
Notably, there are also strong associations between angiogenesis and immune cells. VEGF plays a key role in angiogenesis, a highly complex process that is essential for tumor growth. Studies have shown that VEGF has a significant prognostic role by affecting the metastatic potential of tumors and by correlating with responses to treatment and survival (38,39). Two signaling pathways play important roles in the growth and metastatic potential of human CRCs, including the VEGF and EGFR pathways. EGF is one of the natural ligands of the EGFR, which is a transmembrane tyrosine kinase receptor critical for normal cell proliferation and differentiation. An increased level of EGFR seems to be an important factor driving the aggressive behavior of cancer cells (40). Many studies have shown a relationship between high EGFR levels and high-grade tumors and poor prognosis (41). In line with this, some experimental data has shown that inhibition of the EGFR pathway is associated with increased immune infiltration in solid cancers (42). In this regard, it has also been reported that blocking the EGFR signaling pathway facilitates the activation of immune cells and their recruitment to tumor sites via the production of several cytokines and chemokines (42). This is particularly relevant for NK cells, as studies have shown that the use of a blocking anti-EGFR monoclonal antibody stimulates these innate immune effector lymphocytes and induces antibody-dependent cell cytotoxicity. At the same time, the refractory effect of the tumor against this biological compound may be explained by the induction of mechanisms enacted by the tumor to evade immune responses.
Among the different immune cells, tumor-associated macrophages (TAMs) deserve special attention. Macrophages are widely distributed in the body, and function both as inflammatory cells, engaged in recognition, ingestion, and degradation of cellular debris, foreign material, and pathogens, as well as tissue resident cells, by contributing to maintaining homeostasis in tissues. (43). This is also the case of the resident macrophages of the liver, Kupffer cells (KCs), found lining the walls of the sinusoids. KCs are crucial for clearing the blood entering the sinusoids from pathogens and dangerous endogenous compounds, and are also endowed with high scavenger ability, important for their role in iron recycling from senescent red blood cells (44). Due to their surveillance functions, liver resident macrophages have a tolerogenic and immunosuppressive phenotype in homeostasis. On the other hand, liver macrophages also hold a central and important role during both acute and chronic liver injuries. The heterogeneity in the liver macrophage population relies also on one of the most important shifting paradigms about the origin of hepatic macrophages (44). In fact, it has long been assumed that tissue macrophages originate from circulating monocytes, but it is now increasingly clear that some tissue resident macrophages, including KCs, are long-lived and self-renewing cells, able to persist independently from the embryo into adulthood (45). Therefore, considering that the liver harbors approximately 80% of the macrophages in the whole body, and is anyway patrolled by blood monocytes, liver macrophages represent a promising target for innovative therapies in liver diseases (46). In a recent work, Grossmann et al. showed that targeting of CCR2+ inflammatory monocytes differentiating to intratumor macrophages resulted in restored anti-tumor immunity and increased overall survival in a preclinical model of metastatic CRC (47). This result is sharply in contrast to the positive prognostic function of TAMs in CLM documented in human studies and suggests that the scenario is very complex (48). Such discrepancies could arise from the different monocyte subsets considered. Distinct subsets of monocytes can differentiate in intrahepatic macrophages, giving rise to TAMs with protumor or antitumor functions. For instance, circulating intermediate monocytes (CD14++/CD16+) have been shown to contribute to the response to chemotherapy and antiangiogenic cancer treatment (49).
To date, limited data is available on the role of TAMs in CLM patients. The density of TAMs correlates with survival in primary liver tumor, such as hepatocellular carcinoma (50) and in colo-rectal liver metastases (48). In this work, in particular, a higher density of TAMs at the invasive margin was associated with a better outcome for CLM patients, both as longer disease-free survival and overall survival. Both TAM density and TAM-related markers (e.g., CD68) have been found to correlate with patient prognosis (51,52). Similar data has been recently proposed for patients with cholangiocarcinoma (53). Interestingly, TAMs may express PDL-1, thus acting as immune-suppressor agents, suggesting that they could contribute to the mechanism of action of immunotherapies with anti-PD1 or PDL-1 (54). Therefore, the density of TAMs could be used as a surrogate of therapeutic effects of such immunotherapy. It is commonly known that TAMs are highly plastic and integrate multiple signals to shape their response (55). In the injured liver, macrophages can rapidly change their phenotype depending on the hepatic microenvironment (56). Thus, an imbalance between subtypes of TAMs, for instance M2-type versus M1-type in cancer patients, should be seen as an important element of study in cancer research. The identification of microenvironmental factors and corresponding transcriptional networks underlying macrophage polarization might lead to novel therapeutic strategies. Reshaping of macrophage functions and polarization has, in fact, been suggested in different ways. For instance, Liu et al. (57) showed that silica nanoparticles could trigger the release of cytokines that could polarize macrophages towards an M1-like phenotype. The modulation of macrophage differentiation and in particular the restoration from M2-like to M1-like could be an effective way to control the immune microenvironment in CLM patients. Taken together, the progress in understanding macrophage heterogeneity in liver diseases, and in CLM patients, strongly suggest that we will soon be able to translate these findings into novel therapies in clinical practice.
Key elements also emerge from the genetics of the disease. In a large cohort of colorectal tumors, a gene-gene correlation network was built to understand which genes might be associated with the anti-tumor response (58). Many different genes were identified, but interestingly, most of them were linked to the host immune functionality (T and B cell activation, inflammatory response, T and B cell differentiation, adhesion- and migration-associated chemokines and activation of NK cells), which convincingly suggest that the immune system deserves further attention. Consistent with this, Pitroda et al. (59) recently reported an elegant classification on 134 consecutive resections for CLM that integrated three molecular subtypes matched with the clinical risk stratification. The most favorable subtype was the so-called immune type, which corresponded to those metastases with a high density of CD3+ and CD8+ cells in the peritumor and intratumor compartments with minimal fibrosis. These CLM were associated with some specific mutations, such as POLE, ARID2, EBF1 and CDK12, which were found predictive of the cytotoxic immune response (60). Taken together, the current knowledge suggests that the identification of favorable and unfavorable subtypes of CLM patients, both for genetic mutations and for complex alterations of the tumor microenvironment, is one of the ways to provide the most effective and customizable therapeutic options that might be associated with durable survival. This data and results support how the host immune system represents an emerging hallmark of cancer, which should not be left to exploratory translational research anymore, while it should be routinely considered in clinical practice. However, this data gives the idea that we are still far from the decoding the immune landscape of CLM patients.
Conclusions
Understanding the interactions between immune and cancer cells in CLM in general, and in individual CLM patients, should be a priority in cancer research. The final endpoint is to unearth the basics in order to design approaches arming the immune system against cancer, with the aim to achieve durable survival after surgery and systemic therapy. Many variables can act as confounding factors in the analyses, and many others can hinder the battle conducted by the immune system. Such research should be conducted in synergy between hepatobiliary surgeons, medical oncologists and immunologists to offer an individualized approach, which will likely allow decoding the clinical, pathological, biological and immunological intrigue of CLM patients.
Acknowledgments
This work was supported by AIRC – Associazione Italiana per la Ricerca sul Cancro.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Cucchetti A, Ferrero A, Cescon M, et al. Cure model survival analysis after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2015;22:1908-14. [Crossref] [PubMed]
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677-83. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- Pico de Coana Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med 2015;21:482-91. [Crossref] [PubMed]
- Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15:235-47. [Crossref] [PubMed]
- Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017;17:286-301. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Gupta R, Sinha S, Paul RN. The impact of microsatellite stability status in colorectal cancer. Curr Probl Cancer 2018;42:548-59. [Crossref] [PubMed]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 2009;361:2449-60. [Crossref] [PubMed]
- Mlecnik B, Van den Eynde M, Bindea G, et al. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J Natl Cancer Inst 2018.110. [PubMed]
- Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol 2009;10:877-84. [Crossref] [PubMed]
- Tian T, Olson S, Whitacre JM, et al. The origins of cancer robustness and evolvability. Integr Biol (Camb) 2011;3:17-30. [Crossref] [PubMed]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54-62. [Crossref] [PubMed]
- Hudspeth K, Donadon M, Cimino M, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun 2016;66:40-50. [Crossref] [PubMed]
- Muthuswamy R, Berk E, Junecko BF, et al. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res 2012;72:3735-43. [Crossref] [PubMed]
- Brackett CM, Kojouharov B, Veith J, et al. Toll-like receptor-5 agonist, entolimod, suppresses metastasis and induces immunity by stimulating an NK-dendritic-CD8+ T-cell axis. Proc Natl Acad Sci U S A 2016;113:E874-83. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [Crossref] [PubMed]
- Koebel CM. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450:903-07. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Shankaran V, Ikeda H, Bruce AT, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107-11. [Crossref] [PubMed]
- Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29:610-8. [Crossref] [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [Crossref] [PubMed]
- Hand F, Harmon C, Elliott LA, et al. Depleted polymorphonuclear leukocytes in human metastatic liver reflect an altered immune microenvironment associated with recurrent metastasis. Cancer Immunol Immunother 2018;67:1041-52. [Crossref] [PubMed]
- Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997;182:318-24. [Crossref] [PubMed]
- Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58:3491-4. [PubMed]
- Katz SC, Pillarisetty V, Bamboat ZM, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol 2009;16:2524-30. [Crossref] [PubMed]
- Katz SC, Bamboat ZM, Maker AV, et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol 2013;20:946-55. [Crossref] [PubMed]
- Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009;27:186-92. [Crossref] [PubMed]
- Donadon M, Hudspeth K, Cimino M, et al. Increased Infiltration of Natural Killer and T Cells in Colorectal Liver Metastases Improves Patient Overall Survival. J Gastrointest Surg 2017;21:1226-36. [Crossref] [PubMed]
- Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782-95. [Crossref] [PubMed]
- Amicarella F, Muraro MG, Hirt C, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut 2017;66:692-704. [Crossref] [PubMed]
- Celià-Terrassa T, Kang Y. Metastatic niche functions and therapeutic opportunities. Nat Cell Biol 2018;20:868-77. [Crossref] [PubMed]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015;15:73-86. [Crossref] [PubMed]
- Condamine T, Ramachandran I, Youn JI, et al. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 2015;66:97-110. [Crossref] [PubMed]
- Akbulut H, Altuntas F, Akbulut KG, et al. Prognostic role of serum vascular endothelial growth factor, basic fibroblast growth factor and nitric oxide in patients with colorectal carcinoma. Cytokine 2002;20:184-90. [Crossref] [PubMed]
- De Vita F, Orditura M, Lieto E, et al. Elevated perioperative serum vascular endothelial growth factor levels in patients with colon carcinoma. Cancer 2004;100:270-8. [Crossref] [PubMed]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [Crossref] [PubMed]
- Kopp R, Rothbauer E, Ruge M, et al. Clinical implications of the EGF receptor/ligand system for tumor progression and survival in gastrointestinal carcinomas: evidence for new therapeutic options. Recent Results Cancer Res 2003;162:115-32. [Crossref] [PubMed]
- Hoffmann TK, Schirlau K, Sonkoly E, et al. A novel mechanism for anti-EGFR antibody action involves chemokine-mediated leukocyte infiltration. Int J Cancer 2009;124:2589-96. [Crossref] [PubMed]
- Mantovani A, Marchesi F, Malesci A, et al. Tumour-asso- ciated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399-416. [Crossref] [PubMed]
- Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015;33:643-75. [Crossref] [PubMed]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014;41:21-35. [Crossref] [PubMed]
- Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090-6. [Crossref] [PubMed]
- Grossman JG, Nywening TM, Belt BA, et al. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology 2018;7:e1470729. [Crossref] [PubMed]
- Cavnar MJ, Turcotte S, Katz SC, et al. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann Surg Oncol 2017;24:1835-42. [Crossref] [PubMed]
- Schauer D, Starlinger P, Alidzanovic L, et al. Chemotherapy of colorectal liver metastases induces a rapid rise in intermediate blood monocytes which predicts treatment response. Oncoimmunology 2016;5:e1160185. [Crossref] [PubMed]
- Schneider C, Teufel A, Yevsa T, et al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut 2012;61:1733-43. [Crossref] [PubMed]
- Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predictrs poor prognosis in patients with primary hepato- cellular carcinoma after resection. Hum Pathol 2009;40:381-9. [Crossref] [PubMed]
- Li X, Yao W, Yuan Y, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017;66:157-67. [Crossref] [PubMed]
- Atanasov G, Dietel C, Feldbrügge L, et al. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology 2017;6:e1331806. [Crossref] [PubMed]
- Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014;147:1393-404. [Crossref] [PubMed]
- Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvieroment. Cell 2014;159:1312-26. [Crossref] [PubMed]
- Bartneck M, Fech V, Ehling J, et al. Histidine-rich glycoprotein promotes macrophages activation and inflammation in chronic liver disease. Hepatology 2016;63:1310-24. [Crossref] [PubMed]
- Liu T, Li L, Fu C, et al. Pathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticles. Biomaterials 2012;33:2399-407. [Crossref] [PubMed]
- Mlecnik B, Tosolini M, Charoentong P, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology 2010;138:1429-40. [Crossref] [PubMed]
- Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun 2018;9:1793. [Crossref] [PubMed]
- Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48-61. [Crossref] [PubMed]


