
Techniques for laparoscopic liver parenchymal transection
Introduction
Laparoscopic liver surgery has gained wide acceptance resulting in a paradigm shift of liver surgery (1-3). Technical innovations and accumulation of surgeon’s experience have allowed laparoscopic liver resection (LLR) to become an effective procedure with favorable peri- and post-operative outcomes. While LLR has become standard practice for minor hepatectomy, LLR for major hepatectomy still remains in an exploratory phase (4,5). One of the main reasons, which limit fast expansion of major LLR, remains the difficulty of safely transecting large and deeply located liver parenchymal transection surfaces.
Indeed, the ultimate goal of liver parenchyma transection is to minimize blood loss while obtaining adequate surgical margin clearance for malignancies. Multiple preoperative imaging modalities along with intraoperative ultrasonography findings may contribute to best determining the appropriate cutting line during LLR; however, technical expertise required to obtain adequate exposure along with minimizing and controlling bleeding during liver parenchymal transection remains a challenge for safe LLR, and therefore represents a major concern for hepatobiliary surgeons.
In most cases, the technique of liver parenchymal transection itself is chosen according to surgeon’s preference and “savoir-faire”. In this setting, standardization of practices in order to achieve optimal laparoscopic liver parenchymal transection is currently lacking (6-12). Understanding critical technical issues may allow to define areas for standardization of LLR. Therefore, the aim of this review aims to discuss the technical aspects of laparoscopic liver parenchymal transection.
Preoperative patient evaluation
Patients’ selection is the major factor for successful implementation of any surgical procedure. For LLR, technical difficulty is known to be affected by the procedure itself (e.g., anatomical resection or major hepatectomy) (2,5), tumor factors (e.g., postero-superior segments or involvement of major vessels) (13-15), patients’ characteristics (e.g., elderly, or obesity, history of hepatectomy) (16,17), and underlying liver disease (18,19). Recently, various technical difficulty scores using these variables have been developed (20-23). Likewise, preoperative risk assessment should not be underestimated preoperatively to obtain a successful LLR. Further, multiple imaging modalities such as augmented reality [e.g., three-dimension (3D) computed tomography (24,25)] may allow surgeons to anticipate the meticulous liver anatomy such as location of large hepatic veins and portal veins, possibly leading to avoid accidental injury and better outcomes (25).
Installation
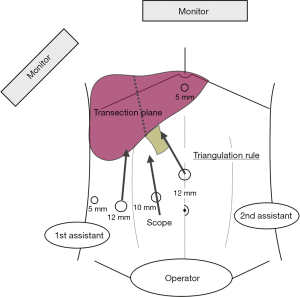
Optimal laparoscopic liver parenchymal transection should align the transection plane with the optical trocar and one operative port on each side to achieve triangulation. Figure 1 shows an example of installation of laparoscopic right hepatectomy and principle of triangulation (26,27).
Patient position
The reverse Trendelenburg position with legs apart is widely accepted for most antero-lateral resections (5,6). In this position, the blood flow returned to the heart is mechanically reduced by gravity, which further helps maintaining low (<5 mmHg) central venous pressure (CVP). The reverse Trendelenburg position also improves exposure by gravitationally shifting visceral structures downwards, away from the liver. On the opposite, semi-/partial/full left lateral or even semi-prone positions, which also use gravity and optimize surgical ergonomics have been developed for LLR of posterosuperior lesions. The latter are considered “difficult locations” for LLR (28). For example, Ikeda et al. developed a new LLR approach using semi-prone position (29,30). The patient is set in the left lateral position and surgeons stand at the left side, with rotating the operating table by 20°–25° from semi-prone position.
Surgeon position and trocar placement
In the French position, the patient is placed in a supine position with the operating surgeon standing between split legs, and is advocated for the vast majority of procedures, such as left lateral sectionectomy or major hepatectomies (26,27). The advantages of this position include the possibility to align the surgeon’s eyes with the optic and monitor, thus respecting the triangulation principle and avoid any shift in angle of vision (Figure 1). This position is also probably more relaxing for surgeons, which is an important issue in long-lasting procedures.
Trocar placements for the assistant are determined on the basis of surgeons’ preference and intraoperative view after positioning the optical trocar. The number of trocars used generally ranges from 4 to 5, while the use of an epigastric port is variable between teams. The optical trocar is most often positioned above the umbilicus but the distance from the umbilicus varies considerably. When approaching posterosuperior segments, a thoracoscopic access using intercostal ports with or without conventional abdominal access may be useful (31,32).
Optical system
A good laparoscopic view is mandatory to safely perform LLR. There are two main types of laparoscopies; forward-oblique viewing laparoscope with 30° or 45°, and flexible laparoscope. Flexible laparoscope may allow for the visualization of several structures (e.g., poster superior segments, root of the right hepatic vein, dome of the right liver), which are more difficult to be visualized using a 30° or 45° laparoscope (28,33). More recently, the 4K resolution technology has been shown to offer up to 4 times better resolutions than the full HD technology but require large screens to be used efficiently. The 3D vision provides a useful depth perception, which allows for enhanced movement precision and has been shown to reduce operation time in LLR (34,35).
Determination of transection plane
Intraoperative ultrasonography is widely used and can clearly and readily identify several important decision-making parameters, such as the extent of tumors and anatomical landmarks (36-38). Especially in anatomical LLR, identification of the hepatic veins and Glissonean pedicles may minimize the risk of vascular injury. The combined use of intraoperative ultrasound with preoperative imaging studies allows determining the appropriate transection line.
When the resection plane cannot be determined simply by ultrasonography alone (in the case of anatomical segmentectomy), intraoperative fluorescence imaging techniques using systemic indocyanine green (ICG) injection helps identifying the boundaries of segments with the associated use of Glissonean pedicles clamping and visualization under a specific camera (6,39-41).
As mentioned earlier, the transection plane should be aligned with optical trocar in accordance with the triangulation rule. Use of retraction, rotating the operating table, handling of transection devices, and change of position of the camera may allow for better exposure of the transection plane.
Basic rules of laparoscopic liver parenchymal transection
Instruments
The devices available for liver parenchymal transection are classified in two main categories: transection devices and energy devices. Transection devices include the ultrasonic scalpel, water-jet, stapler, and cavitron ultrasonic surgical aspirator (CUSA); while energy devices include monopolar and/or bipolar cautery, pre-coagulators, and ultrasonic shears. Energy devices may also be used as transection tools, sealing tools or both.
Table 1 summarizes various instruments for parenchymal transection. According to two recent systematic reviews on LLR (8,9), the transaction methods widely vary depending on the surgeon’s preference. This is possibly explained by two reasons: (I) liver parenchymal transection itself has traditionally been performed according to the surgeon’s preference; and (II) a remarkable increase of new technology with innovative techniques and devices (9). A large retrospective study on more than 5,202 living donors showed that CUSA is used in 86.2% of cases, therefore making it the most appropriate tool for parenchymal transection in order to avoid vessels injury in the open surgery. This trend is similar in the laparoscopic setting. In the 2018 report of the French surgical association focusing on LLR and including more than 4,000 patients, CUSA was the most frequently used tool (64.6% of cases) (42).

Full table
Clips were found to be acceptable for large vessel vascular division. Major vessels (e.g., major hepatic vein or major bile duct) were commonly cut by laparoscopic vascular staplers (11).
How to minimize bleeding from transection plane?
Maintaining a dry operative field to perform safe LLR is of paramount importance. Intermittent clamping using the Pringle maneuver is employed to control hepatic inflow, whereas the maintenance of a relatively low CVP is used to control backflow bleeding from the hepatic vein.
General principles
Patient position, maintenance of pneumoperitoneum between 10–12 mmHg, and low CVP (<5 mmHg), can be associated with low blood loss (43-45). A reverse Trendelenburg position also improves with maintenance of low CVP. The higher the CVP is, the greater the venous engorgement of the liver is, which increases the risk of backflow bleeding during transection. A number of studies have now shown that low CVP anesthesia is well tolerated and that the theoretical risk of gas embolism during laparoscopic approach is very rarely clinically evident (6,7). Besides, maintenance of pneumoperitoneum between 10–12 mmHg itself can contribute to the control of bleeding (2,4,5).
Intermittent pedicular clamping
It is well known that vascular clamping of hepatic inflow and outflow reduces bleeding from liver parenchyma (46-51). Intermittent Pringle maneuver remains the most evidence-based efficient type of clamping for hepatic inflow occlusion and therefore is applied to laparoscopic approach as well (51). By contrast, control of hepatic outflow during LLR requires the use of demanding techniques since the hepatic veins are fragile and vulnerable during parenchymal transection (52). Alternatively, blood loss from the outflow system is strongly correlated with CVP, and therefore maintenance of low CVP has an effect on controlling bleeding.
Intermittent Pringle maneuver can be used during LLR using either an intra- or extracorporeal technique and variants (53-59). Although the intra- corporeal technique has been standardized since its first description, the extra-corporeal technique is a more recently developed one, and has not been described in detail. Recently, Lim et al. compared the outcomes between intra and extracorporeal Pringle maneuver, and they recommended extracorporeal technique because of its rapidity and avoidance of incomplete occlusion of the intrahepatic artery and portal vein (60).
Hemostatic agents for parenchymal transection plane
The use of fibrin-based hemostatic agents and sealants (e.g., TachoSil®, Nycomed Linz, Austria) in open liver resection has gained support through numerous publications citing its efficacy in adjunctive hemorrhage control (61). Three European randomized control trials (RCTs) have already demonstrated the efficacy of hemostatic agents as compared with no agents or argon beam coagulation in liver resection (62-64).
However, the utility of hemostatic agents during the LLR setting remains poorly analyzed. The possible reasons are: (I) laparoscopic positioning of patching agents on transection plane may be technically demanding; (II) LLR is mainly indicated for minor resection for which the use of hemostatic agents may be less relevant; and (III) laparoscopy itself may be associated with enhanced hemostasis of the parenchymal transection plane as a consequence of the increased abdominal pressure generated by the pneumoperitoneum and meticulous surgery brought by the magnified view.
Techniques of bleeding control
Compression for several minutes using small gauze pads (4) and direct clipping for exposed small vessels are simple techniques. When encountering bleeding from a large vessel such as a major hepatic vein, a portal vein, or the inferior vena cava, direct suture of the bleeding source can be attempted after achieving temporary control of the site by applying a clip or grasper (4). In cases of major bleeding during laparoscopic approach, decreasing airway pressure via a brief pause in artificial ventilation is reported to decrease backflow bleeding (65). We do not recommend to increase, even momentarily, pneumoperitoneum pressure in case of hemorrhage, because such high pressure may lead to gas embolism (65).
Techniques for laparoscopic liver parenchymal transection
In this section, we present our techniques of laparoscopic liver parenchymal transection.
Transection of the superficial layer of the liver
Parenchymal transection starts with the opening of both capsule and superficial layer of the liver (up to 2 cm deep) (8), in which no major vessels or bile duct are generally present (Figure 2). This step can be safely performed using ultrasonic shears only (i.e., Harmonic®, Ethicon EndoSurgery, Inc., Cincinnati, OH or Thunderbeat®, Olympus Co., Tokyo, Japan) without pre-coagulation. Doing this, triggering ultrasonic shears before completely closing the device allows limiting bleeding. In selected cases of superficial small tumors requiring wedge resection, we can use ultrasonic shears only without CUSA.
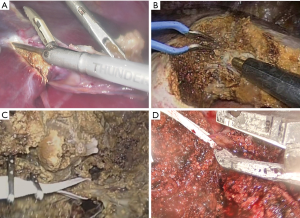
Exposure of intra-parenchymal structures
Transection of deeper parenchyma should be performed with caution and requires meticulous exposure of intra-parenchymal structures (i.e., vessels and bile ducts). To achieve this, both CUSA (CUSA EXcel®: IntegraTM Life Sciences Corporation. NJ, USA) and laparoscopic bipolar cautery for hemostasis are used concomitantly. CUSA can be handled using the right or left hand depending on the axis of the transection plane and surgeon’s preference. Exposing Glissonean branches and/or hepatic veins is clearly in accordance with state of the art open hepatectomy. Pre-coagulation of the cutting plane is not performed to limit the risk of burn injury. The Tissue Select® of the CUSA is a pulsatile mode and appears particularly useful to dissect major hepatic veins in order to reduce the risk of vein injury. This Tissue Select® mode slows down parenchymal transection but allows reducing the risk of vascular injury.
For example, Figure 2B shows liver parenchymal transection during laparoscopic right hepatectomy. The middle hepatic vein should be exposed on the cutting plane, which ensures appropriate resection, and makes division easier and faster because almost no Glissonean branch is present in this cutting plane.
Small vessels (diameter of 2 mm or less) are diathermically sealed using sealing devices (i.e., Thunderbeat®) and then divided. Hemostasis of the resection plane is achieved with bipolar cautery (66). Larger vessels (diameter of 3–7 mm) are divided with sealing devices or clips as appropriate. Significant hepatic veins or Glissonean pedicles are dissected and then taped allowing for traction and good positioning of the clips of suture (Figure 2C). This process allows for preventing clips or suture from untying, breaking, and slipping. Almost all cases are then double clipped using Hem-o-lok® (Weck Closure Systems. NC, USA) and divided by straight scissors. Vascular stapler is used for the division of large structures.
Liberal use of intermittent pedicular clamping
To minimize bleeding from transection plane, intermittent pedicular clamping is used without restriction. The use of an intermittent Pringle maneuver has been reported to have no detrimental effects on postoperative liver function (2,67). Prior to transecting liver parenchyma, vascular tape should be placed around hepatoduodenal ligament by opening the lesser omentum and passing the tape through the foramen of Winslow. This process allows to easily perform the Pringle maneuver and various techniques of intra-corporeal or extra-corporeal pedicular clamping can be used depending on the surgeon’s preference. At our institute we use an extracorporeal clamping technique using a dedicated vascular clamp (Figure 3). This approach is easy, reproducible, quickly usable, effective, and safe and can be applied without assist by surgeons with limited experience in LLR.

Transection of the hepatic outflow
For major hepatectomy, transection of the major hepatic veins is performed with a laparoscopic vascular stapler. The proper identification and isolation of the hepatic veins may be the most difficult aspect of these procedures. For example, in laparoscopic right hepatectomy, the right hepatic vein is gently encircled after transection of the liver parenchyma and then hang up using a dedicated tape (Figure 2D) as mentioned earlier. This process also allows for secure vascular stapling without damaging hepatic veins and misfire.
Recovery from major bleeding; our experience
Figure 4 shows the management of IVC bleeding during right hepatectomy. First of all, it is mandatory to be able to stop the bleeding by immediately occluding the venous injury using a dedicated laparoscopic vascular clamp. Obviously, laparoscopic suturing is more challenging than in open surgery. Considering the significant time from conversion to open approach, however, laparoscopic suturing may be effective. Likewise, we emphasize that massive bleeding should be repaired under laparoscopic approach. Expertise of laparoscopic suturing technique is known to be more difficult than open approach, yet, it is mandatory to control any bleeding, especially on main hepatic veins (2,4).

Conclusions
Liver parenchymal transection continues to be the technical challenge of the pure laparoscopic approach. Optimal determination of the transection line, appropriate use of devices, and better understanding the basic rules of bleeding control allow to perform a cautious transection; which further contributes to safer as well as standardization of LLR.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Cho JY, Han HS, Wakabayashi G, et al. Practical guidelines for performing laparoscopic liver resection based on the second international laparoscopic liver consensus conference. Surg Oncol 2018;27:A5-9. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Jia C, Li H, Wen N, et al. Laparoscopic liver resection: a review of current indications and surgical techniques. Hepatobiliary Surg Nutr 2018;7:277-88. [Crossref] [PubMed]
- Cai X. Laparoscopic liver resection: the current status and the future. Hepatobiliary Surg Nutr 2018;7:98-104. [Crossref] [PubMed]
- Otsuka Y, Kaneko H, Cleary SP, et al. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2015;22:363-70. [Crossref] [PubMed]
- Scatton O, Brustia R, Belli G, et al. What kind of energy devices should be used for laparoscopic liver resection? Recommendations from a systematic review. J Hepatobiliary Pancreat Sci 2015;22:327-34. [Crossref] [PubMed]
- Berber E, Akyuz M, Aucejo F, et al. Initial experience with a new articulating energy device for laparoscopic liver resection. Surg Endosc 2014;28:974-8. [Crossref] [PubMed]
- Buell JF, Gayet B, Han HS, et al. Evaluation of stapler hepatectomy during a laparoscopic liver resection. HPB (Oxford) 2013;15:845-50. [Crossref] [PubMed]
- Dural C, Akyuz M, Yazici P, et al. Safety and efficacy of a new bipolar energy device for parenchymal dissection in laparoscopic liver resection. Surg Laparosc Endosc Percutan Tech 2016;26:21-4. [Crossref] [PubMed]
- Ai JH, Li JW, Chen J, et al. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One 2013;8:e72328. [Crossref] [PubMed]
- Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 2015;22:1288-93. [Crossref] [PubMed]
- Yoon YS, Han HS, Cho JY, et al. Laparoscopic liver resection for centrally located tumors close to the hilum, major hepatic veins, or inferior vena cava. Surgery 2013;153:502-9. [Crossref] [PubMed]
- Cauchy F, Fuks D, Nomi T, et al. Benefits of laparoscopy in elderly patients requiring major liver resection. J Am Coll Surg 2016;222:174-84.e10. [Crossref] [PubMed]
- Uchida H, Iwashita Y, Saga K, et al. Benefit of laparoscopic liver resection in high body mass index patients. World J Gastroenterol 2016;22:3015-22. [Crossref] [PubMed]
- Harada N, Maeda T, Yoshizumi T, et al. Laparoscopic liver resection is a feasible treatment for patients with hepatocellular carcinoma and portal hypertension. Anticancer Res 2016;36:3489-97. [PubMed]
- Cai X, Liang X, Tunan T, et al. Liver cirrhosis grading Child-Pugh B: a Goliath to challenge in laparoscopic liver resection? Prior experience and matched comparisons. Hepatobiliary Surg Nutr 2015;4:391-7. [PubMed]
- Halls MC, Berardi G, Cipriani F, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg 2018;105:1182-91. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of laparoscopic liver resection: proposal for a new classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Hasegawa Y, Wakabayashi G, Nitta H, et al. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc 2017;31:5356-63. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Radtke A, Sotiropoulos GC, Molmenti EP, et al. Computer-assisted surgery planning for complex liver resections. Ann Surg 2010;252:876-83. [Crossref] [PubMed]
- Nakayama K, Oshiro Y, Miyamoto R, et al. The Effect of Three-Dimensional Preoperative Simulation on Liver Surgery. World J Surg 2017;41:1840-7. [Crossref] [PubMed]
- Goumard C, Farges O, Laurent A, et al. An update on laparoscopic liver resection: The French Hepato-Bilio-Pancreatic Surgery Association statement. J Visc Surg 2015;152:107-12. [Crossref] [PubMed]
- Soubrane O, Schwarz L, Cauchy F, et al. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles: The Caudal Approach. Ann Surg 2015;261:1226-31. [Crossref] [PubMed]
- Teo JY, Kam JH, Chan CY, et al. Laparoscopic liver resection for posterosuperior and anterolateral lesions-a comparison experience in an Asian centre. Hepatobiliary Surg Nutr 2015;4:379-90. [PubMed]
- Ikeda T, Yonemura Y, Ueda N, et al. Pure laparoscopic right hepatectomy in the semi-prone position using the intrahepatic Glissonian approach and a modified hanging maneuver to minimize intraoperative bleeding. Surg Today 2011;41:1592-8. [Crossref] [PubMed]
- Ikeda T, Mano Y, Morita K, et al. Pure laparoscopic hepatectomy in semiprone position for right hepatic major resection. J Hepatobiliary Pancreat Sci 2013;20:145-50. [Crossref] [PubMed]
- Ogiso S, Conrad C, Araki K, et al. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg 2015;262:358-65. [Crossref] [PubMed]
- Chiow AK, Lewin J, Manoharan B, et al. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford) 2015;17:299-303. [Crossref] [PubMed]
- Hong SK, Shin E, Lee KW, et al. Pure laparoscopic donor right hepatectomy: perspectives in manipulating a flexible scope. Surg Endosc 2019;33:1667-73. [Crossref] [PubMed]
- Velayutham V, Fuks D, Nomi T, et al. 3D visualization reduces operating time when compared to high-definition 2D in laparoscopic liver resection: a case-matched study. Surg Endosc 2016;30:147-53. [Crossref] [PubMed]
- Kawai T, Goumard C, Jeune F, et al. 3D vision and maintenance of stable pneumoperitoneum: a new step in the development of laparoscopic right hepatectomy. Surg Endosc 2018;32:3706-12. [Crossref] [PubMed]
- Santambrogio R, Opocher E, Ceretti AP, et al. Impact of intraoperative ultrasonography in laparoscopic liver surgery. Surg Endosc 2007;21:181-8. [Crossref] [PubMed]
- Lai EC, Tang CN, Ha JP, et al. The evolving influence of laparoscopy and laparoscopic ultrasonography on patients with hepatocellular carcinoma. Am J Surg 2008;196:736-40. [Crossref] [PubMed]
- Araki K, Conrad C, Ogiso S, et al. Intraoperative ultrasonography of laparoscopic hepatectomy: key technique for safe liver transection. J Am Coll Surg 2014;218:e37-41. [Crossref] [PubMed]
- Sakoda M, Ueno S, Iino S, et al. Anatomical laparoscopic hepatectomy for hepatocellular carcinoma using indocyanine green fluorescence imaging. J Laparoendosc Adv Surg Tech A 2014;24:878-82. [Crossref] [PubMed]
- Ishizawa T, Zuker NB, Kokudo N, et al. Positive and negative staining of hepatic segments by use of fluorescent imaging techniques during laparoscopic hepatectomy. Arch Surg 2012;147:393-4. [Crossref] [PubMed]
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. [Crossref] [PubMed]
- Scatton O, Vibert E. Chirurgie hépatique par laparoscopie. Rapport présenté au 120e congrès français de chirurgie 2018. Paris: John Libbey Eurotext, 2018.
- Schmandra TC, Mierdl S, Hollander D, et al. Risk of gas embolism in hand-assisted versus total laparoscopic hepatic resection. Surg Technol Int 2004;12:137-43. [PubMed]
- Jayaraman S, Khakhar A, Yang H, et al. The association between central venous pressure, pneumoperitoneum, and venous carbon dioxide embolism in laparoscopic hepatectomy. Surg Endosc 2009;23:2369-73. [Crossref] [PubMed]
- Coelho FF, Kruger JA, Fonseca GM, et al. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg 2016;8:5-26. [Crossref] [PubMed]
- Ercolani G, Ravaioli M, Grazi GL, et al. Use of vascular clamping in hepatic surgery: lessons learned from 1260 liver resections. Arch Surg 2008;143:380-7. [Crossref] [PubMed]
- Elias D, Lasser P, Debaene B, et al. Intermittent vascular exclusion of the liver (without vena cava clamping) during major hepatectomy. Br J Surg 1995;82:1535-9. [Crossref] [PubMed]
- Lesurtel M, Selzner M, Petrowsky H, et al. How should transection of the liver be performed? a prospective randomized study in 100 consecutive patients comparing four different transection strategies. Ann Surg 2005;242:814-22; discussion 822-3. [Crossref] [PubMed]
- Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-11; discussion 711-3. [Crossref] [PubMed]
- Weiss MJ, Ito H, Araujo RL, et al. Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: no impact on survival or hepatic recurrence. Ann Surg Oncol 2013;20:285-94. [Crossref] [PubMed]
- Gurusamy KS, Sheth H, Kumar Y, et al. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev 2009.CD007632. [PubMed]
- Wang HB, Zhang Y, Hu YD, et al. Retrograde laparoscopic resection of left side of the liver: a safe and effective way. Surg Endosc 2016;30:3848-53. [Crossref] [PubMed]
- Patriti A, Ceccarelli G, Bartoli A, et al. Extracorporeal Pringle maneuver in robot-assisted liver surgery. Surg Laparosc Endosc Percutan Tech 2011;21:e242-4. [Crossref] [PubMed]
- Rotellar F, Pardo F, Bueno A, et al. Extracorporeal tourniquet method for intermittent hepatic pedicle clamping during laparoscopic liver surgery: an easy, cheap, and effective technique. Langenbecks Arch Surg 2012;397:481-5. [Crossref] [PubMed]
- Okuda Y, Honda G, Kurata M, et al. Useful and convenient procedure for intermittent vascular occlusion in laparoscopic hepatectomy. Asian J Endosc Surg 2012;6:100e103.
- DuaMM, WorhunskyDJ, HwaK, et al. Extracorporeal Pringle for laparoscopic liver resection. Surg Endosc 2014;29:1348e1355.
- Piardi T, Lhuaire M, Memeo R, et al. Laparoscopic Pringle maneuver: how we do it? Hepatobiliary Surg Nutr 2016;5:345-9. [Crossref] [PubMed]
- Inoue Y, Suzuki Y, Fujii K, et al. Laparoscopic hepatic resection using extracorporeal Pringle maneuver. J Laparoendosc Adv Surg Tech A 2018;28:452-8. [Crossref] [PubMed]
- Huang JW, Su WL, Wang SN. Alternative Laparoscopic Intracorporeal Pringle Maneuver by Huang's Loop. World J Surg 2018;42:3312-5. [Crossref] [PubMed]
- Lim C, Osseis M, Lahat E, et al. Extracorporeal Pringle Maneuver During Laparoscopic and Robotic Hepatectomy: Detailed Technique and First Comparison with Intracorporeal Maneuver. J Am Coll Surg 2018;226:e19-25. [Crossref] [PubMed]
- Simo KA, Hanna EM, Imagawa DK, et al. Hemostatic Agents in Hepatobiliary and Pancreas Surgery: A Review of the Literature and Critical Evaluation of a Novel Carrier-Bound Fibrin Sealant (TachoSil). ISRN Surg 2012;2012:729086. [PubMed]
- Briceño J, Naranjo A, Ciria R, et al. A prospective study of the efficacy of clinical application of a new carrier-bound fibrin sealant after liver resection. Arch Surg 2010;145:482-8. [Crossref] [PubMed]
- Frilling A, Stavrou GA, Mischinger HJ, et al. Effectiveness of a new carrier-bound fibrin sealant versus argon beamer as haemostatic agent during liver resection: a randomised prospective trial. Langenbecks Arch Surg 2005;390:114-20. [Crossref] [PubMed]
- Fischer L, Seiler CM, Broelsch CE, et al. Hemostatic efficacy of TachoSil in liver resection compared with argon beam coagulator treatment: an open, randomized, prospective, multicenter, parallel-group trial. Surgery 2011;149:48-55. [Crossref] [PubMed]
- Kobayashi S, Honda G, Kurata M, et al. An experimental study on the relationship among airway pressure, pneumoperitoneum pressure, and central venous pressure in pure laparoscopic hepatectomy. Ann Surg 2016;263:1159-63. [Crossref] [PubMed]
- Mbah NA, Brown RE, Bower MR, et al. Differences between bipolar compression and ultrasonic devices for parenchymal transection during laparoscopic liver resection. HPB (Oxford) 2012;14:126-31. [Crossref] [PubMed]
- Dua MM, Worhunsky DJ, Hwa K, et al. Extra-corporeal Pringle for laparoscopic liver resection. Surg Endosc 2015;29:1348-55. [Crossref] [PubMed]
- Yoh T, Cauchy F, Soubrane O. Extracorporeal clamping technique using a dedicated vascular clamp. Asvide 2019;6:333. Available online: http://www.asvide.com/watch/33018
- Yoh T, Cauchy F, Soubrane O. Management of bleeding from vena cave during right hepatectomy. Asvide 2019;6:334. Available online: http://www.asvide.com/watch/33019


