Histamine regulation of pancreatitis and pancreatic cancer: a review of recent findings
Introduction
Pancreas
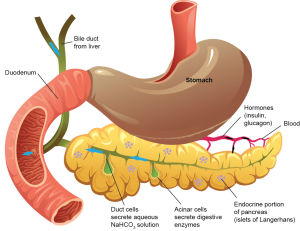
The pancreas plays a key role in humans serving as both an endocrine and exocrine gland in the digestive system (1). The pancreas extends across the abdomen, and has an enlarged head region as well as a tail portion. Located behind the stomach and partially connected to the duodenum, the pancreas acts to aid in digestion as well as adjusting gastrointestinal hormone levels. The exocrine portion of the pancreas utilizes zymogens and bicarbonate in order to assist in digestion and neutralization, respectively (2). As to be expected, the endocrine pancreas aids in regulating hormone levels to allow for metabolic homeostasis.
Comprising part of the small intestinal tract, the pancreas is made up of different epithelial cell types, which have very specific functions. Various genes have been associated with the formation and regulation of the pancreas such as Sox 9, Neurog3, and Ptf1a (1). The exocrine region of the pancreas is composed of duct cells and acinar cells which function to produce zymogens (2). Duct cells deliver zymogens and bicarbonate for activation in the duodenum, and subsequently digestion of food (2). In the endocrine portion of the pancreas there are large collections of epithelial cells called islets of Langerhans (3). The islets of Langerhans contain several types of hormone-secreting cells including α, β, γ, ε, and pancreatic polypeptide-secreting cells (3). β cells have received much attention in research because these cells produce insulin, and are implicated various forms of diabetes (3). Figure 1 depicts a cartoon schematic of the pancreas, pancreatic cells and surrounding organs (open access, no copyright).
Pancreatitis
There exist two divisions of pancreatitis: acute and chronic, with autoimmune pancreatitis falling under the chronic distinction (4). Pancreatitis can arise due to damage of the pancreas, infection, as a result of alcoholism and smoking or due to genetic mechanisms (2). Acute pancreatitis is diagnosed in 210,000 Americans every year, and can range from mild to lethal in severity, with 20% of cases resulting in death caused by necrotizing disease (5). The condition is caused by the pancreas using its own proteases to digest itself. It remains unknown whether or not trypsin and cholecystokinin are directly responsible for this autodigestive damage (6). Acute pancreatitis results in symptoms of epigastric pain, vomiting, and nausea, along with markedly increased amylase and lipase levels (6). To determine the prognosis, the systemic inflammatory response (SIRS) and the Bedside Index for Severity of Acute Pancreatitis (BISAP) tests are employed (5). Currently, there are not many treatments to combat the disease itself, but supportive treatments such as fluid resuscitation, and enteral feeding help to maintain health in the patient (5).
Chronic pancreatitis typically originates from acute pancreatitis although not all patients continue on to develop chronic pancreatitis. This recurrent form of pancreatitis is marked by fibrosis, loss of islet and acinar cells in the pancreas, and inflammation (7). Patients who develop acute pancreatitis by smoking or consuming alcohol are more likely to develop the chronic form of pancreatitis (7). Diagnosing chronic pancreatitis can be attained through various imaging tests (MRI, EUS, CT), and patients typically present with great abdominal pain similar to that of acute pancreatitis (7). Like acute pancreatitis, treating and managing chronic pancreatitis is very difficult; Many times even after therapy, patients still retain symptoms (7). In order to manage chronic pancreatitis, abstinence from alcohol, pancreatic enzymes, analgesics, or sometimes narcotics and opioids are employed (7).
Autoimmune pancreatitis typically presents in middle-aged and elderly males, accompanied with obstructive jaundice, diabetes mellitus, and epigastric discomfort (8). Three criteria are used to diagnose autoimmune pancreatitis; Enlargement of the pancreas and narrowing of the main pancreatic duct, elevated levels of autoantibodies, and lymphoplasmacytic infiltration and fibrosis (8). Although rare, it has also been found that autoimmune pancreatitis can be misdiagnosed as, or found in conjunction with pancreatic cancer. These findings make it rather difficult for clinicians to determine the true diagnosis of the patient, so tests to differentiate the conditions must be used (4).
Pancreatic cancer
According to the American Cancer Society, in 2013, there will be 45,220 cases of pancreatic cancer diagnosed in US, with 38,460 dying of this devastating cancer (9). Pancreatic cancer remains one of the deadliest cancer types, with a five-year survival rate of about 5% (10). Pancreatic cancer is notorious for being asymptomatic, which then allows the disease a greater ability to metastasize to other organs before it is ever diagnosed. This particular type of cancer arises due to several factors, including environmental, genetic, and pathological causes (11). Specifically, a history of smoking, increased body mass index, family history of pancreatic cancer, alcoholism, pancreatitis, and diabetes mellitus are all factors, which increase the risk of obtaining pancreatic cancer (10). There are various forms of pancreatic cancer, which affect both the endocrine and exocrine pancreas systems. The most common cancerous tumor in the pancreas is invasive ductal adenocarcinoma, and typically retains the title of pancreatic cancer (11). Neuroendocrine tumors in the pancreas may also arise, but are much less prevalent (12). Neuroendocrine tumors result due to an excess in pancreatic hormone levels, and treatment must be established to correct the excess in that particular hormone, as well as possibly identifying the presence of an inherited disease that caused this excess (12). Currently, due to the asymptomatic nature of pancreatic cancer, early diagnosis remains a challenge. However, once the diagnosis is made, patients typically undergo various treatments such as chemotherapy, chemoradiotherapy, possibly surgical resection of the tumor, as well as other supportive therapies (4,9,10,12).
Histamine
The biogenic monoamine histamine is one of the most intensely studied molecules in the biological system (13). Histamine is known to induce broad spectrum of biological activities including cell proliferation, differentiation, regulation of gastrointestinal function, and modulation of immune responses (13). Histamine is a low molecular weight amine synthesized exclusively by L-HDC that is expressed in numerous cells throughout the body including gastric-mucosa, parietal, and mast cells (14). After histamine is formed by HDC it is rapidly stored or degraded (15).
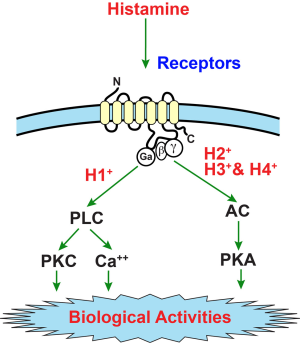
Histamine exerts its biological effects by interacting with four G protein-coupled (GPCR) receptors, i.e., H1HR, H2HR, H3HR, and H4HR (15). Activating or inhibiting the HRs triggers downstream signaling pathways to elicit immune-modulatory and pro-inflammatory cellular responses (15). H1HRs main signal is induced by ligand binding and activation of phospholipase C-generating inositol 1, 4, 5-triphosphate and 1, 2-diacylglycerol (DAG) leading to increased cytosolic Ca2+ (13,16,17). The H2HR is coupled to adenylate cyclase and to phosphoinositide secondary messenger system via separate GTP-dependent mechanisms (18,19). Histamine is a strong stimulant of cAMP accumulation in many cells, and H2HR-dependent signaling of histamine is typically mediated through cAMP (18,19). In contrast, H3HR activation triggers inhibition of cAMP formation, accumulation of Ca2+ and stimulation of mitogen-activated protein kinase (MAPK) (20,21). H4HR is expressed in many areas of the body including intestinal tissue, basophils, and mast cells (13,22). Similar to H3HR, H4HR signaling mechanisms triggers an inhibition of adenylyl cyclase and downstream of cAMP response elements as well as activation of MAPK (22,23). Figure 2 depicts the generally acknowledged signaling of the histamine/histamine receptor axis [used with permission from Shahid, et al., “Histamine, Histamine Receptors, and Their Role in Immunomodulation: An Updated Systematic Review” The Open Immunology Journal, 2009;2:9-41 (open access journal)].
The role of histamine during biliary damage and biliary cancer has been extensively studied (14,15,17,18,20,21,23). Because the biliary tract and pancreas share similar pathological, phenotypical and biological features (24) it is likely that histamine, the histamine receptors and the synthesis of histamine by HDC are all important in the regulation of pancreatic diseases like pancreatitis and pancreatic carcinoma.
Histamine and pancreatitis
As stated above, while most cases of acute pancreatitis are non-progressive, recurrent episodes may lead to chronic pancreatitis, which is also a very challenging disease (25). As the pathophysiological causes of acute and chronic pancreatitis gradually become revealed to us, it is hopeful that their treatments will become more successful. However, the biological basis of pain and the mechanisms underlying the pathogenesis of acute and chronic pancreatitis is still poorly understood (26-28). Recent findings indicate that the activation of granulocytes and macrophages in pancreatitis results in the release of a number of cytokines and inflammatory mediators and an important inflammatory mediator, the mast cell, secretes histamine as well as other chemotactic molecules and inflammation activators (29-31). Mast cells have been implicated in the pathogenesis of pain in other conditions and some have hypothesized that mast cells and histamine secretion play a role in the pain of chronic pancreatitis, which is characterized by mononuclear inflammatory cell infiltration (27,32-34). Interestingly, it has been shown that humans with painful chronic pancreatitis have an increased number of pancreatic mast cells compared to those with painless chronic pancreatitis (27).
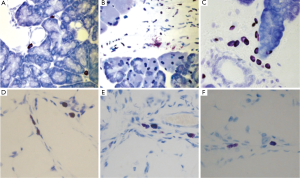
Because histamine is produced predominantly by mast cells, it is important to consider studies involving mast cell activation and pancreatitis to analyze the resultant effects of histamine during the course of this threatening disease. It has also been argued that increased circulating histamine levels worsen distant organ injury, especially if derived from pancreatic mast cells in cases of acute and chronic pancreatitis (28,35,36). In an attempt to study the activation of pancreatic mast cells and the effects of mast cell inhibition on the activation of peritoneal and alveolar macrophages during acute pancreatitis, a recent study suggested that pancreatic mast cells are significant triggers of local and SIRS in the early phases of acute pancreatitis (37). These authors confirmed that pancreatitis resulted in increased levels of circulating histamine in plasma in both the pancreas and lung. The inhibition of mast cell degranulation with cromolyn sodium also resulted in a reduction in pancreatic myeloperoxidase (MPO) activity, indicating that this treatment reduced pancreatic inflammation (37). Several mediators released by mast cells, including histamine are increased shortly after (a few minutes) the induction of pancreatitis in their experimental models. In addition to this, administration of mast cell inhibitors results in a reduction of the local and SIRS and prevents changes in endothelial cells and vascular permeability (37). As histamine has been confirmed to be a potent vasodilator, histamine may possibly be an important factor to study in increased vascular permeability in pancreatic inflammation (28,38). There is a very large population of mast cells that resides in the periacinar space, pancreatic interstitium and mesentery and these mast cells degranulate early in acute pancreatitis (39). In three models of necrotizing disease, local increases were noted of mast-cell mediators, histamine being one of them (39). In addition, water immersion-induced stress was shown to result in the conversion of hyperstimulation mild pancreatitis into necrohaemorrhagic disease, similarly to when histamine or dimethyl PGE2 were added in a duct hyperpermeability model of mild pancreatitis (40). A similar pattern of increase in plasma histamine has been recorded in pancreatitis models upon exposure to water immersion stress (40,41). As acute pancreatitis can be fatal when it advances to systemic inflammation and multi-organ failure, Kempuraj et al., desired to study a mouse model of pancreatic duct ligation-induced acute pancreatitis that is associated with systemic inflammation and substantial mortality (28). These authors found using an Enzyme-Linked Immunosorbant Assay (ELISA) for in vivo mouse duct ligation-induced acute pancreatitis models, plasma histamine concentrations were increased. In concurrence with these findings, it has also been speculated that increased circulating histamine levels originating from activated pancreatic mast cells are implicated in the development of acute lung injury during acute pancreatitis (28,35,36). Zhao et al., demonstrates that pancreatitis-associated lung injury is an early-occurring and severe complication that involves a number of inflammatory cells and their products in the initiation and progress of the condition (36). While higher plasma levels of histamine were determined, the intraperitoneal administration of cromolyn (a mast cell stabilizer) reduced pancreatitis-induced systemic increase of histamine after one hour. Cromolyn was also found to prevent pancreatitis-induced pulmonary endothelial barrier dysfunction after 6 hours (36). The authors hypothesize that mast cells and histamine may play an important role in the activation of leukocytes during the initiation of pancreatitis-associated lung injury by altering phenotypes of adhesion molecules (36). Figure 3 shows immunohistochemistry for mast cells (marked by toluidine blue) in the pancreas and lung after induction of pancreatitis (B and E) and after cromolyn treatment (C and F). Degranulating mast cells were observed in the pancreas after pancreatitis induction (B) and cromolyn treatment inhibited mast cell degranulation (Reused with permission from World Journal of Gastroenterology “Pancreatic and pulmonary mast cells activation during experimental acute pancreatitis” 2010;16:3411-7).
In clinical trials, controlling histamine levels by way of receptor inhibition or mast cell stabilization has yielded mixed results. In a retrospective analysis of pancreatic exocrine insufficiency (PEI), Sander-Struckmeier et al., aimed to determine whether the efficacy of pancrealipase/pancreatin may be affected by the concomitant use of proton pump inhibitors (PPIs)/histamine-2 receptor antagonists (H2RAs) (42). PEI, which is a deficiency or absence of digestive enzyme secretion in the duodenum, is associated with many pancreatic disorders such as cystic fibrosis and chronic pancreatitis. Analyzed data from a number of clinical trials in patients having PEI indicates that the efficacy of pancrelipase/pancreatin is not affected by concomitant PPI/H2RA use and concurs with the treatment guidelines’ recommendation that acid suppression is not routinely mandated with pancreatic enzyme replacement therapy (42). Early administration of protease inhibitors has commonly been employed for the therapy of acute pancreatitis in Japan. Kohsaki et al., state that a number of clinical trials have failed to show the clinical effects of protease inhibitors and H2 receptor antagonist during the treatment of acute pancreatitis and these authors argue that well-organized clinical studies should be undertaken to assess the relative therapeutic value of these agents for acute pancreatitis (43). However, according to Abdel Aziz et al., non-enteric-coated enzyme preparations along with acid suppression (histamine-2 blockers or PPIs) have at least a modest effectiveness in treating pain caused by chronic pancreatitis and may be worth a trial in patients with less advanced disease diagnosis (44). Clearly, more work needs to be performed to fully understand the role of histamine and histamine receptors (H1-H4HRs) on the treatment strategies of pancreatitis.
Histamine and pancreatic cancers
Pancreatic cancer is the leading cause of cancer-related deaths worldwide due to its aggressive nature (45). For this reason, it is of great importance that we ascertain the interrelations of pancreatic cancer and discover new therapeutics to help combat this devastating disease. HDC, which converts histidine to histamine, has been an important area of study recently due to histamine’s known ability to accelerate cancerous cells into cell cycle arrest (45). In normal pancreatic islet cells, HDC is predominantly found in glucagon cells, but in pancreatic tumors, HDC was found in all types of islet cells: glucagon-, insulin-, somatostatin-, pancreatic polypeptide- and serotonin-producing enterochromaffin cells (46). Over expression of HDC has been demonstrated in various pancreatic cancer cells, but one study found that 79% (19/24) of the evaluated pancreatic tumors showed HDC expression (46,47). Based on this study, we can define HDC as an indicator of endocrine differentiation and, therefore, a potential diagnostic tool in pancreatic cancer (46).
Various other studies have been performed to evaluate how histamine carries out cellular proliferation via its G-protein coupled histamine receptors (H1-H4HR). Previous research performed on the PANC-1 cell line, which is derived from human pancreatic carcinoma and contains a mutated P53, demonstrated that these cells over express H1HR and H2HR (45). These PANC-1 cells can also secrete histamine into the extracellular medium where it can act as an autocrine or paracrine growth factor to regulate cellular proliferation through the binding of H1 and H2 histamine receptors (45,48). When bound to H1HR, histamine has been shown to induce PANC-1 proliferation by up regulating nerve growth factor (NGF) secretion and mRNA expression (49). These effects can be negated by pyrilamine, the H1HR antagonist, which further proves the increased proliferative effect of histamine (49). Histamine or an agonist binding to H1HR has also proven to influence PANC-1 cells into metastasis due to a decrease in cellular adhesion; which is associated with an increase in matrix metalloprotease 2 (MMP2) activity (48,50). In contrast, when H1HR and H2HR were blocked using specific receptor antagonists there was an increase in adhesion and, therefore, a decrease in cellular motility (48). Activation of H2HR in PANC-1 cells tends to have the opposite effect of H1HR activation. A study by Cricco et al., states that H2HR activation, through the binding of histamine, generates partial cellular differentiation of the PANC-1 cell line and stimulates cAMP production to inhibit proliferation (45). Bcl-2, an anti-apoptotic regulator protein, may be involved in this regulation of cAMP (45). This H2HR activation also inhibited PANC-1 cell growth by moving the cells into G0/G1 phase arrest (45). Furthermore, the expression of proliferating cell nuclear antigen (PCNA) and Bax, a pro-apoptotic factor, were decreased through H2HR modulation (45,51). Additional research done on the various histamine receptors has proven that H3HR stimulation increases cellular proliferation by regulating the cell cycle, but that H4HR stimulation diminishes pancreatic tumor growth (52,53). The pathological and biological functions of H3HR and H4HR activation in pancreatic tumors are vague and require further investigation in order to be clearly defined.
Currently, mast cells have been of great interest in the studies of pancreatic cancer as well (54). Mast cells are ubiquitous and found throughout all tissues of the human body and contain many cytoplasmic granules that, when stimulated, are able to release considerable quantities of histamine into the surrounding microenvironment (54). Mast cells have other roles, but their release of histamine is what draws them into the interest of this review. In general, mast cell infiltration is increased in cases of pancreatic cancer when compared to normal pancreatic tissue (55). This same study demonstrated how PANC-1 cells, when in the presence of mast cell conditioned media, were accompanied with an influx of mast cells; Which leads to increased pancreatic cancer cell migration, proliferation, and invasion (55). Conversely, this infiltration did not have an effect on normal pancreatic tissues (55). This increase in mast cell number can be an indicator of higher-grade pancreatic adenocarcinoma and, therefore, can be correlated with poorer prognosis for the patient (54,55). The exact pathological and physiological role of mast cells and histamine secretion in pancreatic carcinoma is unclear and requires further research.
Clinical studies in pancreatic cancer
Currently, 5-6% of pancreatic cancer patients with non-resectable disease have an estimated survival rate of 5-years, but chemotherapy resistant patients have a median survival time of <6 months (56,57). Due to its poor prognosis and aggressive nature pancreatic cancer has prompted many research facilities to perform clinical trials to help determine a productive treatment method for this disease. For the past 10-15 years the main form of chemotherapy treatment for advanced and metastatic cancer was the use of gemcitabine, an anti-metabolite (56). Generally, gemcitabine is a favorable treatment in patients that have a poor performance status (56). Five recent clinical trials tested the efficacy of gemcitabine by administering it to different sets of patients; four trials administered it as a fixed dose rate of 10 mg/m2/min, while one trial administered it as a standard infusion rate over 30 minutes (56,58-61). The data from all five of these trials gave a median response rate (RR) of 23%, a median progression-free survival (PFS) of 4 months, and an overall survival (OS) of 6 months (56).
Research has suggested that there may be a survival benefit in first-line treatment when erlotinib, a tyrosine kinase inhibitor, is combined with gemcitabine (56,62). One trial treated patients in a 1:1 ratio with either a combination of erlotinib and gemcitabine or gemcitabine together with a placebo (62). The results from this study concluded that OS was significantly prolonged with the erlotinib/gemcitabine treatment compared to gemcitabine with the placebo (6.24 vs. 5.91 months, respectively) (62). Overall first-year survival was also greater with the combined treatment (23% vs. 17%), but there tended to be more adverse side effects with the combined treatment (erlotinib and gemcitabine) when compared to gemcitabine with the placebo (62).
Other studies have suggested the use of gemcitabine in conjunction with platinum agents as a potential treatment in the first-line setting of advanced pancreatic cancer (56,63-65). When analyzing the results from gemcitabine with platinum agents there tended to be an improvement in RR and PFS (P=0.006 and 0.059, respectively), but no significant improvement in OS (P=0.1) when compared to other methods of treatment (56). Gemcitabine is not the only compound that has been suggested to be used with platinum agents. Some research has suggested the use of the pyrimidine analog, 5-fluoruracil (5-FU) with platinum agents as well (56). Recently, 8 trials studied the effectiveness of 5-FU with oxiplatin, a platinum based alkylating agent, and 2 trials tested 5-FU with cisplatin, a platinum containing drug that cross-links DNA (56,62,66-72). When analyzing the data from these 8 clinical trials, the 5-FU combined with a platinum agent had a median PFS of 2.9 months and a median OS of 5.7 months (56,62,66-72). When compared to the treatments stated earlier, it seems as if the combination of 5-FU with platinum containing agents is less efficient in terms of survival benefit (56).
Future studies and concluding statement
Throughout this article we have aimed to highlight the characteristics of pancreatitis, pancreatic cancer, and histamine’s role in the progression and development of these diseases. When viewing current literature that discusses the involvement of histamine in various forms of pancreatitis we can see that histamine and mast cell secretion tightly regulate inflammation, which can ultimately lead to endothelial cell destruction. Being able to block this pro-inflammatory signaling pathway via mast cell degranulation inhibition may decrease the damaging effects that histamine is able to enact on the pancreas. Histamine has also been labeled as a vasodilator in pancreatitis, but its exact function in this area requires further investigation. In terms of clinical trials, some work on the treatment of acute pancreatitis using protease inhibitors and an H2 receptor antagonist were ineffective in terms of prevention and replacement therapy, but were successful in relieving pain in early developed acute pancreatitis. Clinical trials on histamine influence in pancreatitis are few and far between and thus require additional studies. Histamine’s regulation of pancreatic cancer is more convoluted and confusing than it is in pancreatitis in general. When working through its H1HR and H3HR receptors histamine has demonstrated pro-proliferative and metastatic abilities in the PANC-1 cell line, but when bound to H2HR or H4HR histamine has proven to be anti-proliferative through G0/G1 cell cycle arrest, the diminishment of tumor growth, and generation of partial cellular differentiation. Recent clinical trials on pancreatic cancer have been valuable in terms of generating treatment options that successfully help to prolong life, but no current trials have been performed with histamine-, mast cell-, or histamine receptor-related therapies. For this reason, clinical studies on the manipulation of histamine through various signaling molecules, such as mast cells, HDC, and H1-H4HR, need to be developed to help determine if this area of study could be beneficial to the lives of future patients. The future of histamine in prevention, diagnosis and therapy of pancreatic diseases is unknown and open to evaluation and experimentation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Arda HE, Benitez CM, Kim SK. Gene regulatory networks governing pancreas development. Dev Cell 2013;25:5-13. [PubMed]
- Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology 2013;144:1292-302. [PubMed]
- Sandovici I, Hammerle CM, Ozanne SE, et al. Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes. Cell Mol Life Sci 2013;70:1575-95. [PubMed]
- Chandrasegaram MD, Chiam SC, Nguyen NQ, et al. A case of pancreatic cancer in the setting of autoimmune pancreatitis with nondiagnostic serum markers. Case Rep Surg 2013;2013:809023.
- Baron T. Managing severe acute pancreatitis. Cleve Clin J Med 2013;80:354-9. [PubMed]
- Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 2013;144:1180-93. [PubMed]
- Forsmark CE. Management of chronic pancreatitis. Gastroenterology 2013;144:1282-91.e3.
- Kamisawa T, Okazaki K, Kawa S. Diagnostic criteria for autoimmune pancreatitis in Japan. World J Gastroenterol 2008;14:4992-4. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013. [Epub ahead of print]. [PubMed]
- Sakorafas GH, Tsiotos GG. Molecular biology of pancreatic cancer: potential clinical implications. BioDrugs 2001;15:439-52. [PubMed]
- Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol 2012;26:737-53. [PubMed]
- Pino-Ángeles A, Reyes-Palomares A, Melgarejo E, et al. Histamine: an undercover agent in multiple rare diseases? J Cell Mol Med 2012;16:1947-60. [PubMed]
- Francis H, DeMorrow S, Venter J, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut 2012;61:753-64. [PubMed]
- Francis H, Meng F, Gaudio E, et al. Histamine regulation of biliary proliferation. J Hepatol 2012;56:1204-6. [PubMed]
- Bryce PJ, Mathias CB, Harrison KL, et al. The H1 histamine receptor regulates allergic lung responses. J Clin Invest 2006;116:1624-32. [PubMed]
- Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 2008;295:C499-513. [PubMed]
- Francis HL, Demorrow S, Franchitto A, et al. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest 2012;92:282-94. [PubMed]
- Monczor F, Fernandez N, Riveiro E, et al. Histamine H2 receptor overexpression induces U937 cell differentiation despite triggered mechanisms to attenuate cAMP signalling. Biochem Pharmacol 2006;71:1219-28. [PubMed]
- Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest 2007;87:473-87. [PubMed]
- Francis H, Onori P, Gaudio E, et al. H3 histamine receptor-mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res 2009;7:1704-13. [PubMed]
- Dunford PJ, Williams KN, Desai PJ, et al. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol 2007;119:176-83. [PubMed]
- Meng F, Han Y, Staloch D, et al. The H4 histamine receptor agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis. Hepatology 2011;54:1718-28. [PubMed]
- Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int 2010;60:419-29. [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [PubMed]
- Drewes AM, Krarup AL, Detlefsen S, et al. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut 2008;57:1616-27. [PubMed]
- Hoogerwerf WA, Gondesen K, Xiao SY, et al. The role of mast cells in the pathogenesis of pain in chronic pancreatitis. BMC Gastroenterol 2005;5:8. [PubMed]
- Kempuraj D, Twait EC, Williard DE, et al. The novel cytokine interleukin-33 activates acinar cell proinflammatory pathways and induces acute pancreatic inflammation in mice. PLoS One 2013;8:e56866. [PubMed]
- Chang DZ, Ma Y, Ji B, et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res 2011;17:7015-23. [PubMed]
- Dyduch G, Kaczmarczyk K, Okoń K. Mast cells and cancer: enemies or allies? Pol J Pathol 2012;63:1-7. [PubMed]
- Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol 2004;114:21-7. [PubMed]
- Demir IE, Schorn S, Schremmer-Danninger E, et al. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One 2013;8:e60529. [PubMed]
- Esposito I, Friess H, Kappeler A, et al. Mast cell distribution and activation in chronic pancreatitis. Hum Pathol 2001;32:1174-83. [PubMed]
- Wood JD. Visceral pain: spinal afferents, enteric mast cells, enteric nervous system and stress. Curr Pharm Des 2011;17:1573-5. [PubMed]
- Nathan C. Points of control in inflammation. Nature 2002;420:846-52. [PubMed]
- Zhao X, Dib M, Wang X, et al. Influence of mast cells on the expression of adhesion molecules on circulating and migrating leukocytes in acute pancreatitis-associated lung injury. Lung 2005;183:253-64. [PubMed]
- Lopez-Font I, Gea-Sorlí S, de-Madaria E, et al. Pancreatic and pulmonary mast cells activation during experimental acute pancreatitis. World J Gastroenterol 2010;16:3411-7. [PubMed]
- Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol 2006;147 Suppl 1:S127-35. [PubMed]
- Braganza JM. Mast cell: pivotal player in lethal acute pancreatitis. QJM 2000;93:469-76. [PubMed]
- Yamaguchi H, Kimura T, Nawata H. Does stress play a role in the development of severe pancreatitis in rats? Gastroenterology 1990;98:1682-8. [PubMed]
- Huang ZL, Mochizuki T, Watanabe H, et al. Biphasic elevation of plasma histamine induced by water immersion stress, and their sources in rats. Eur J Pharmacol 1998;360:139-46. [PubMed]
- Sander-Struckmeier S, Beckmann K, Janssen-van Solingen G, et al. Retrospective analysis to investigate the effect of concomitant use of gastric acid-suppressing drugs on the efficacy and safety of pancrelipase/pancreatin (CREON®) in patients with pancreatic exocrine insufficiency. Pancreas 2013;42:983-9. [PubMed]
- Kohsaki T, Nishimori I, Onishi S. Treatment of acute pancreatitis with protease inhibitor, H2 receptor antagonist and somatostatin analogue. Nihon Rinsho 2004;62:2057-62. [PubMed]
- Abdel Aziz AM, Lehman GA. Current treatment options for chronic pancreatitis. Curr Treat Options Gastroenterol 2007;10:355-68. [PubMed]
- Cricco G, Martín G, Medina V, et al. Histamine inhibits cell proliferation and modulates the expression of Bcl-2 family proteins via the H2 receptor in human pancreatic cancer cells. Anticancer Res 2006;26:4443-50. [PubMed]
- Tanimoto A, Matsuki Y, Tomita T, et al. Histidine decarboxylase expression in pancreatic endocrine cells and related tumors. Pathol Int 2004;54:408-12. [PubMed]
- Rivera ES, Cricco GP, Engel NI, et al. Histamine as an autocrine growth factor: an unusual role for a widespread mediator. Semin Cancer Biol 2000;10:15-23. [PubMed]
- Cricco G, Núñez M, Medina V, et al. Histamine modulates cellular events involved in tumour invasiveness in pancreatic carcinoma cells. Inflamm Res 2006;55 Suppl 1:S83-4. [PubMed]
- Wang ZY, Ding Y, Miki T, et al. Nerve growth factor and receptors are significantly affected by histamine stimulus through H1 receptor in pancreatic carcinoma cells. Mol Med Rep 2010;3:103-9. [PubMed]
- Folgueras AR, Pendás AM, Sánchez LM, et al. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 2004;48:411-24. [PubMed]
- Hersey P, Zhang XD. Overcoming resistance of cancer cells to apoptosis. J Cell Physiol 2003;196:9-18. [PubMed]
- Coruzzi G, Adami M, Pozzoli C. Role of histamine H4 receptors in the gastrointestinal tract. Front Biosci 2012;4:226-39. [PubMed]
- Cricco GP, Mohamad NA, Sambuco LA, et al. Histamine regulates pancreatic carcinoma cell growth through H3 and H4 receptors. Inflamm Res 2008;57 Suppl 1:S23-4. [PubMed]
- Hodges K, Kennedy L, Meng F, et al. Mast cells, disease and gastrointestinal cancer: A comprehensive review of recent findings. Transl Gastrointest Cancer 2012;1:138-50. [PubMed]
- Strouch MJ, Cheon EC, Salabat MR, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res 2010;16:2257-65. [PubMed]
- Rahma OE, Duffy A, Liewehr DJ, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 2013;24:1972-9. [PubMed]
- Yutani S, Komatsu N, Yoshitomi M, et al. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol Rep 2013. [Epub ahead of print]. [PubMed]
- Demols A, Peeters M, Polus M, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 2006;94:481-5. [PubMed]
- Fortune BE, Li X, Kosuri KV, et al. Fixed-dose-rate gemcitabine in combination with oxaliplatin in patients with metastatic pancreatic cancer refractory to standard-dose-rate gemcitabine: a single-institute study. Oncology 2009;76:333-7. [PubMed]
- Kozuch P, Grossbard ML, Barzdins A, et al. Irinotecan combined with gemcitabine, 5-fluorouracil, leucovorin, and cisplatin (G-FLIP) is an effective and noncrossresistant treatment for chemotherapy refractory metastatic pancreatic cancer. Oncologist 2001;6:488-95. [PubMed]
- Reni M, Cereda S, Mazza E, et al. PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) regimen as second-line therapy in patients with progressive or recurrent pancreatic cancer after gemcitabine-containing chemotherapy. Am J Clin Oncol 2008;31:145-50. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Heinemann V, Labianca R, Hinke A, et al. Increased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicenter study. Ann Oncol 2007;18:1652-9. [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [PubMed]
- Sultana A, Tudur Smith C, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer 2008;99:6-13. [PubMed]
- Gebbia V, Maiello E, Giuliani F, et al. Second-line chemotherapy in advanced pancreatic carcinoma: a multicenter survey of the Gruppo Oncologico Italia Meridionale on the activity and safety of the FOLFOX4 regimen in clinical practice. Ann Oncol 2007;18 Suppl 6:vi124-7. [PubMed]
- Mitry E, Ducreux M, Ould-Kaci M, et al. Oxaliplatin combined with 5-FU in second line treatment of advanced pancreatic adenocarcinoma. Results of a phase II trial. Gastroenterol Clin Biol 2006;30:357-63. [PubMed]
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [PubMed]
- Pelzer U, Stieler J, Roll L, et al. Second-line therapy in refractory pancreatic cancer. results of a phase II study. Onkologie 2009;32:99-102. [PubMed]
- Saif MW. New developments in the treatment of pancreatic cancer. Highlights from the “44th ASCO Annual Meeting”. Chicago, IL, USA. May 30-June 3, 2008. JOP 2008;9:391-7. [PubMed]
- Tsavaris N, Kosmas C, Skopelitis H, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs 2005;23:369-75. [PubMed]
- Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009;101:1658-63. [PubMed]