
Chills and fever as the first presentation of hepatic perivascular epithelioid cell tumor
A 70-year-old female was admitted to the hospital with a history of chills and fever (with a high temperature of 37.5 °C) for 17 days. No obvious anomalies were detected on physical examination. Laboratory tests indicated white blood cells (6.96×109/L), neutrophil percentage (65%), C-reactive protein, and procalcitonin (0.766 ng/mL) in the normal range. The tests for liver function were normal, with Child-Pugh grade A, and the patient tested negative for hepatitis B virus infection. Tumor markers, such as carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), and carbohydrate antigen 19-9 (CA19-9) were normal. All other test indicators were normal. Contrast-enhanced ultrasonography (CEUS) (Figure 1A) detected a solid mass in the right posterior lobe of the liver measuring 7.9 cm × 6.4 cm, with a clear boundary and an uneven hypoechoic region. A small blood flow signal was detected, with an annular enhancement pattern and centripetal filling. Hepatic enhanced computed tomography (CT) and magnetic resonance imaging (MRI) showed a lumpy mixed-density shadow (approximately 6.8 cm × 6.2 cm) in the right posterior lobe of the liver. A circular low-density shadow and a calcified nodule were detected at the edge, and medium heterogeneous enhancement was observed in the arterial phase of the enhanced scan. A transient high-perfusion area was observed around the arterial phase (Figure 1B,C,D,E,F). Positron emission tomography-computed tomography (PET-CT) (Figure 1G) showed a heterogeneous density mass in the right posterior lobe of the liver, measuring approximately 7.2 cm × 5.6 cm × 7.6 cm, with uneven internal density, a fat density shadow, and a calcified nodule at the edge, with an uneven increase in fluorodeoxyglucose (FDG) uptake.
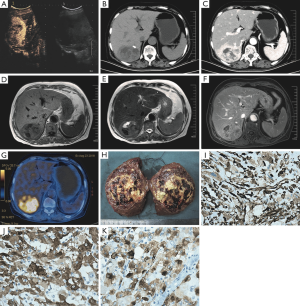
The mass was 8 cm × 7 cm × 4 cm in size, and the surface was pale yellow, brownish yellow, and dark red in different areas, with soft and partly necrotic tissue, an intact capsule, and an unclear boundary (Figure 1H). Histologically, the tumor was composed of cytoplasmic epithelioid cells and thick-walled blood vessels, the cells were polygonal and proliferated around blood vessels.
Immunohistochemistry results showed that tumor cells were positive for vimentin, melan-A, Hmb-45, CD10 (focal +), ki-67 (~10%), and CD117 (partial +) (Figure 1I,J,K), and negative for smooth muscle actin (SMA).
The patient took oral Jinkehuaier granules regularly during follow-up. The patient was followed-up for 6 months without symptoms and had a good quality of life. During this period, CT showed no evidence of tumor recurrence or metastasis.
Discussion
Liver perivascular epithelioid cell tumors (PEComas) are rare mesenchymal tumors (1), and were first described by Yamasaki in 2000 (2). Most patients with PEComa of the liver have no characteristic clinical symptoms, and a small number of patients may suffer from abdominal pain or abdominal discomfort. This case is the first reported case presenting with chills and fever.
In this patient, differentiating between liver abscess and liver PEComa was challenging. A hepatic abscess was considered a possible diagnosis because of the patient’s clinical symptoms of chills, fever, and blood sedimentation speed changes. In addition, the CT and MRI characteristics of patients with liver neoplasm are similar to those of liver abscess, namely a single lesion, uneven mixed density on CT scan, a visible ring with low density at the edge, long T1 and T2 signals on MRI, presence of a honeycomb pattern, and edge edema with high perfusion reinforcement. PET-CT also suggested liver abscess according to the characteristics of the FDG metabolism of the tumor. However, despite presenting with a fever, it was not persistently high, and the test indexes of leukocytes, neutrophil percentage, C-reactive protein, and procalcitonin were not high. Liver function tests were normal.
The differential diagnosis of tumors as hepatic abscess needs further discussion. CEUS indicated that the tumor was inhomogeneous and hypoechoic, and blood flow signals were detected. The tumor showed an annular centripetal filling type of inhomogeneous enhancement, which suggested liver malignancy. The CT and MRI features of the tumor were not completely consistent with the imaging features of liver abscess. There were obvious arteriotrophic vessels in the arterial phase of the tumor, indicating that the tumor was not a simple abscess. Moreover, the abscess wall generally presents with a regular ring shape, smooth contour, and uniform thickness, and the enhancement shows a typical “double ring sign”, whereas in this patient, the low-density ring edge of the hepatoma showed uneven thickness, and the inner edge was uneven. Although the enhancement was obvious, there was no “double ring sign”. The plain MRI scan of a hepatic abscess also shows an obvious “halo ring sign”, whereas tumors do not have this feature. Therefore, the liver mass of this patient had features consistent with a diagnosis of malignant tumor.
The preoperative diagnosis of liver PEComa is based on imaging examination. Hepatic PEComa ultrasonography shows non-uniform echo with low echo, a blood flow signal around the tumor, and abundant parenchyma. CEUS showed concentric filling enhancement (3). On plain CT scan, liver PEComa often presents with an uneven low-density shadow, and a fat density shadow can be seen in tumors containing adipose tissue (4). Enhanced CT shows uneven enhancement, and usually large and tortuous vascular shadows are observed. Plain MRI scan shows long T1 and T2 signals, and the enhancement features of the lesion are similar to those of CT. PET-CT usually shows heterogeneous radioactive concentration in lesions, and delayed imaging radioactive concentration is reduced to varying degrees, a feature that can be used to distinguish PEComa from other liver malignancies (5). The imaging characteristics of this patient were consistent with the literature reports, although unique characteristics were also detected. The preoperative imaging diagnostic accuracy of PEComa in the liver is relatively low (6). The final diagnosis depends on postoperative pathology.
In summary, this is the first reported case of liver PEComa presenting with chills and fever. CT and MRI findings included a marginal low-density ring with continuous enhancement, which should be differentiated from hepatic abscess.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kirnap M, Ozgun G, Moray G, et al. Perivascular epithelioid cell tumor outgrowth from the liver. Int J Surg Case Rep 2018;53:295-8. [Crossref] [PubMed]
- Yamasaki S, Tanaka S, Fujii H, et al. Monotypic epithelioid angiomyolipoma of the liver. Histopathology 2000;36:451-6. [Crossref] [PubMed]
- Tan Y, Xie X, Lin Y, et al. Hepatic epithelioid angiomyolipoma: clinical features and imaging findings of contrast-enhanced ultrasound and CT. Clin Radiol 2017;72:339.e1-e6. [Crossref] [PubMed]
- Chen W, Liu Y, Zhuang Y, et al. Hepatic perivascular epithelioid cell neoplasm: a clinical and pathological experience in diagnosis and treatment. Mol Clin Oncol 2017;6:487-93. [Crossref] [PubMed]
- Sun L, Sun X, Li Y, et al. The role of (18)F-FDG PET/CT imaging in patient with malignant PEComa treated with mTOR inhibitor. Onco Targets Ther 2015;8:1967-70. [Crossref] [PubMed]
- Yang X, Li A, Wu M. Hepatic angiomyolipoma: clinical, imaging and pathological features in 178 cases. Med Oncol 2013;30:416. [Crossref] [PubMed]


