Eat more carrots? Dampening cell death in ethanol-induced liver fibrosis by β-carotene
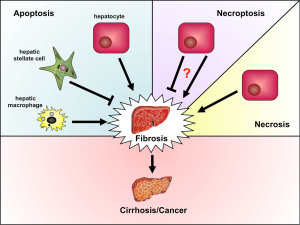
Long-term ethanol abuse leads to alcoholic liver disease (ALD), which represents one of the principal causes of liver damage in humans. Liver tissue from patients with ALD show fatty degeneration, termed steatosis, accompanied by liver cell death and inflammatory infiltrates, while simultaneously markers of lipid peroxidation increase in serum (1-4). Liver fibrogenesis leading to cirrhosis and tumor development represent the end-stage of ALD with subsequent liver failure being the main cause of death among ALD patients. One of the key mechanisms during ethanol-induced liver injury is induction of oxidative stress and production of reactive oxygen species (ROS). Ethanol uptake stimulates expression and activity of the cytochrome P450 2E1 (CYP2E1) causing oxidative stress in liver mitochondria resulting in the release of ROS and increased lipid peroxidation (5,6). Ethanol also triggers liver inflammation through increasing the gut permeability for endotoxins (7). The latter activate hepatic macrophages (Kupffer cells, KC) to produce proinflammatory mediators like tumor necrosis factor (TNF) or interleukin-6 (IL-6) as well as chemokines attracting other leukocytes, mainly neutrophils (8). Activated KC and neutrophils not only contribute to the oxidative stress in the liver by producing ROS, activated KC also exert profibrogenic actions by activating hepatic stellate cells (HSC) that produce collagen (9). One of the main results of oxidative stress in the liver is hepatocyte apoptosis through cell intrinsic mechanisms along with extrinsic mechanisms like TNF receptor-mediated apoptosis, which in turn promotes liver fibrogenesis (Figure 1) (4). In ALD patients hepatocyte apoptosis positively correlates with disease severity (10) and in animal studies treatments with antioxidants were able to reduce hepatocyte death (11,12). Since current therapies for ALD are very limited, antioxidants may display promising treatment options.
In this issue of Hepatobiliary Surgery and Nutrition (HBSN), Peng et al. demonstrate the antioxidative capacity of β-carotene in a rat model of chronic alcohol feeding. At two different concentrations, supplementation with β-carotene reduced ethanol-induced liver damage and lipid peroxidation; accordingly, rats showed lower levels of CYP2E1 and thiobarbituric acid-reactive substance (TBARS) in the liver. This was accompanied by decreased hepatic inflammation and lower TNF levels in liver and serum. The group also showed for the first time that β-carotene, apart from its antioxidant capacity, has an anti-apoptotic effect on the liver in vivo. Addition of β-carotene during ethanol diet markedly reduced expression of apoptosis-related markers like cytochrome c and caspase enzymes, whereas anti-apoptotic proteins like Bcl-2 and Bcl-XL were enhanced compared to ethanol feeding alone.
As excessive apoptosis in the liver usually induces compensatory mechanisms that often lead to development of organ fibrosis or even tumorigenesis, these findings might make β-carotene a prospective target for treatment of ALD. However, the exact mechanisms how and on which cell types β-carotene acts in the liver remained elusive. While hepatocyte apoptosis is often considered as a trigger for fibrogenesis and tumor development (13) and apoptosis of hepatic macrophages was shown to provoke persistent inflammation in chronic injury (14), other cell death mechanisms such as necroptosis in hepatocytes, which have not been investigated in this manuscript, might reduce inflammation and prevent hepatocarcinogenesis (15). Moreover, apoptosis of other cell types, like collagen-producing HSC, might even be helpful to prevent hepatic fibrosis (Figure 1). HSC are the major extracellular matrix producing cells in the liver, and activation of these cells drives fibrosis development. Consistently, we recently showed that natural killer (NK) or gamma/delta T cell-induced apoptosis of HSC was able to reduce experimental hepatic fibrogenesis in mice (16). Thus, it is of utmost importance to further investigate the anti-apoptotic effects of β-carotene in the liver and determine which cell populations and which cell death pathways are targeted by this carotenoid.
The beauty of this study is the simple, straightforward approach of orally supplementing β-carotene during ethanol consumption. However, one must remain cautious before translating these interesting and promising observations into clinical scenarios. Importantly, previous studies had indicated rather harmful effects of long-term β-carotene treatment. Baboons chronically fed ethanol showed enhanced hepatotoxic effects when they additionally received β-carotene (17). Moreover, two clinical studies on smokers showed a higher incidence of lung cancer in subjects treated with β-carotene compared to the placebo group (18,19).
Another aspect that should be carefully considered before translating the findings from the current study by Peng and coworkers into clinical trials are “patient-relevant endpoints”. By using the rat model of ethanol-feeding, only mild fibrosis developed. However, patients with ALDs are threatened by severe alcoholic hepatitis with a high risk of mortality, fibrosis and cirrhosis as long-term consequences of chronic inflammation as well as hepatocellular carcinoma (HCC) after decades of chronic liver injury (20). Thus, preclinical interventional studies should be designed addressing the impact of β-carotene supplementation on any of these “hard end-points”. It should be also considered that hepatocyte apoptosis might be an important mechanism of clearing (pre-) malignant hepatocytes, thus possibly preventing or alleviating the development of HCC (21).
Finally, it is currently unclear, whether the observed anti-oxidant and anti-apoptotic properties of β-carotene are specific for alcohol-induced liver damage or represent a more general mechanism during chronic liver injury. As fibrogenesis and tumor development are common consequences of all chronic liver diseases, often involving hepatocyte apoptosis, an oral treatment option capable of reducing liver damage through limiting cell death would be highly warranted in patients suffering from chronic liver diseases. Thus, we believe the encouraging data presented by Peng and coworkers should promote further research on β-carotene and ALD, especially considering cell-type specific effects, potential influences on organ fibrosis and tumorigenesis, dose finding and translational approaches for human disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Aleynik SI, Leo MA, Aleynik MK, et al. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res 1998;22:192-6. [PubMed]
- Clot P, Tabone M, Aricò S, et al. Monitoring oxidative damage in patients with liver cirrhosis and different daily alcohol intake. Gut 1994;35:1637-43. [PubMed]
- Ishak KG, Zimmerman HJ, Ray MB. Alcoholic liver disease: pathologic, pathogenetic and clinical aspects. Alcohol Clin Exp Res 1991;15:45-66. [PubMed]
- Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci 2005;10:3093-9. [PubMed]
- Fang C, Lindros KO, Badger TM, et al. Zonated expression of cytokines in rat liver: effect of chronic ethanol and the cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology 1998;27:1304-10. [PubMed]
- Girre C, Lucas D, Hispard E, et al. Assessment of cytochrome P4502E1 induction in alcoholic patients by chlorzoxazone pharmacokinetics. Biochem Pharmacol 1994;47:1503-8. [PubMed]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2004;286:G881-4. [PubMed]
- Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol 2004;287:G310-4. [PubMed]
- Pradere JP, Kluwe J, De Minicis S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 2013;58:1461-73. [PubMed]
- Natori S, Rust C, Stadheim LM, et al. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol 2001;34:248-53. [PubMed]
- Kono H, Arteel GE, Rusyn I, et al. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med 2001;30:403-11. [PubMed]
- Kono H, Rusyn I, Bradford BU, et al. Allopurinol prevents early alcohol-induced liver injury in rats. J Pharmacol Exp Ther 2000;293:296-303. [PubMed]
- Vucur M, Reisinger F, Gautheron J, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep 2013;4:776-90. [PubMed]
- Karlmark KR, Zimmermann HW, Roderburg C, et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010;52:1769-82. [PubMed]
- Liedtke C, Bangen JM, Freimuth J, et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology 2011;141:2176-87. [PubMed]
- Hammerich L, Bangen JM, Govaere O, et al. Chemokine receptor CCR6-dependent accumulation of γδ T-cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 2013. [Epub ahead of print]. [PubMed]
- Leo MA, Kim C, Lowe N, et al. Interaction of ethanol with beta-carotene: delayed blood clearance and enhanced hepatotoxicity. Hepatology 1992;15:883-91. [PubMed]
- Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 1996;88:1550-9. [PubMed]
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994;330:1029-35. [PubMed]
- Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol 2013;28:77-84. [PubMed]
- Vucur M, Roderburg C, Bettermann K, et al. Mouse models of hepatocarcinogenesis: what can we learn for the prevention of human hepatocellular carcinoma? Oncotarget 2010;1:373-8. [PubMed]


