Cholangiocarcinoma: increasing burden of classifications
Introduction
Cholangiocarcinoma (CCA) is the second most frequent primitive liver malignancy representing approximately 7-10% of the total. CCA may originate, at any portion of the biliary tree, from the neoplastic proliferation of cholangiocytes, the epithelial cells lining bile ducts (1-8). CCA is a very heterogeneous cancer, from any point of view, including epidemiology, risk factors, morphology, pathology, molecular pathology, modalities of growth and clinical features (1-10). Given this heterogeneity, a uniform classification respecting the epidemiologic, pathologic and clinical needs is currently lacking. In the last few years, a huge number of different classifications have been proposed. In this manuscript we discussed the different proposed classifications of CCA in relation with recent advances in pathophysiology and biology of this cancer.
CCA classification based on anatomical location
According to different guidelines, CCA is classified in intrahepatic (IH-CCA) and extrahepatic (EH-CCA), the second order bile ducts representing the separation point. EH-CCA is further divided in perihilar (Klatskin tumour) and distal, the separation point being located downstream or upstream the insertion of the cystic duct according to the World Health Organization (WHO) or Union for International Cancer Control (UICC) classification (1-4,6). The classification based on anatomical location is certainly valid from an iconographic point of view and, indeed, is largely used in the medical literature (11). However, this classification is biased by a number of pitfalls. First, diagnosis of CCA frequently occurs at an advanced stage and this is especially true for the perihilar CCA, where, discrimination between the intrahepatic or extrahepatic location results hard and challenging. Indeed, in cancer registries, as much as 30-40% of CCAs are classified as NOS (i.e., site non otherwise specified) (11). Second, small bile ducts and ductules are also present in the perihilar liver parenchyma. Therefore, perihilar CCA may originate either from these small ducts or from large perihilar ducts but this cannot be discriminated by a classification based on anatomical location. On the light of recent advances (see above) the cells of origin of CCA is acquiring increasing pathological and clinical relevance (12-24). Third, recent studies demonstrated how from a pathological and molecular point of view, no difference exists between EH-CCA and the IH-CCA originated from large intrahepatic bile ducts and, therefore, the distinction between these two forms of CCA is loosing relevance (20,25).
A bulk of medical literature deals with the difference between IH- and EH-CCA in terms of epidemiology and risk factors. With the exception of data from Denmark, studies investigating CCA epidemiology indicate a progressive worldwide increase of incidence and mortality for IH-CCA, whereas EH-CCA seems to be stable or slightly decreasing (6,26-29). However, the epidemiologic studies showing these differences between IH- and EH-CCA could have been biased by the misclassification of perihilar CCA as IH- rather EH-CCA. According to Welzel TM (6), the misclassification of perihilar CCA caused, in epidemiologic surveys, an overestimation of IH-CCA (and underestimation of EH-CCA) incidence of approx. 10-15%. In contrast, according to a more recent study (30) the misclassification of perihilar CCA based on the old ICD codes, leads to a complete misleading of epidemiologic data. These controversies, that justify the search for alternative classifications of CCA, also concern studies on risk factors. According to these studies, there are risk factors common to both IH- and EH-CCA including biliary diseases such as choledochal cysts, cholangitis/primary sclerosing cholangitis (PSC), secondary biliary cirrhosis, choledocholithiasis, hepatolithiasis, cholecystitis, and liver flukes. These are pathologic conditions primarily affecting large intra-hepatic (IH) bile ducts and/or extra-hepatic (EH) bile ducts and, therefore, it is not surprising that they may favor the incidence of both IH- and EH-CCA. In contrast, cholelithiasias and cholecystectomy primarily affect EH bile ducts. Consequently, these conditions are recognized risk factors mainly for EH-CCA. Finally, parenchymal liver diseases including, chronic viral and non-viral liver diseases, have the interlobular bile ducts, bile ductules and the canals of Hering as their primary targets of damage. In fact, interlobular bile ducts, bile ductules and the canals of Hering are the main players involved in ductular reaction, a phenomenon shared by all these pathologies. Consequently, these conditions are recognized risk factors mainly for IH-CCA. How the studies concerning the distribution of risk factors between IH- and EH-CCA are influenced by the misclassification of CCA is a matter of debate.
CCA classification based on macroscopic pattern of growth
By taking into consideration the macroscopic pattern of growth, IH-CCA has been classified, by the Liver Cancer Study Group of Japan (LSCGJ) (31), as mass-forming, periductal infiltrating and intraductal growing. According to the American Joint Committee on Cancer (AJCC) and UICC, a mixed type (periductal infiltrating plus intraductal growing) should be also considered. EH-CCA may growth by following an esophitic pattern, nodular or periductal infiltrating, or an intraductal growing pattern, and this has been specifically considered by the LSCGJ association. In two Italian surveys (28,32), 89-94% of IH-CCA presents as mass-forming type, single mass in 78% of cases. In these studies, 30% of IH-CCAs were associated with positivity of hepatitis virus markers (HBV, HCV) and in 14% of patients in the setting of liver cirrhosis. As far as EH-CCA is concerned, 78% of cases showed a modality of growth of periductal infiltrating + nodular type, associated in 18.6% of cases with positive hepatitis virus markers and in 4.3% with cirrhosis. Few studies tried to investigate the correlates between the macroscopic pattern of growth and clinic-pathologic features. To this regard, Komuta et al. described how the mass forming is a typical modality of presentation of peripheral intrahepatic non mucin (MUC) producing CCA (20). This type of CCA is frequently associated with positivity of hepatitis virus markers. The periductal infiltrating type, IH- or EH-CCA, is frequently a MUC producing CCA while the intraductal growing type IH- or EH- is a papillary/polypoid type of CCA. A part from this information, evidence are lacking for strict correlates between the pattern of growth and pathologic or molecular features and, therefore, this type of classification is not very useful for clinical or epidemiologic purposes.
CCA classification based on microscopic features
A huge number of microscopic forms of CCA have been described. IH-CCA and EH-CCA are usually adenocarcinomas that are well, moderately, or poorly differentiated. Several rare types of IH- or EH-CCA, based on histologic analyses, have been described (1-4,21). Recently, a clear histological distinction between CCAs originating from extrahepatic bile ducts and large intrahepatic bile ducts from one side, and CCA originating from peripheral bile ducts from the other side, has been highlighted. Indeed, Okuda et al. (33) proposed to classify CCA into peripheral and hilar types (33). This proposal of grossly classification is consistent with the microscopic counterpart. In fact, perihilar-CCA and IH-CCA originating from large ducts consist of well/moderate differentiated MUC-producing adenocarcinoma; this being classified as pure MUC-CCA (20). On the other hand, the most peripheral CCA types such as the cholangiolocarcinomas (CLCs) and the IH-CCAs with focal areas of hepatocitic differentiation, classified as mixed-type CCAs, showed a complete histological overlap, the distinction depending from the extension of neoplastic ductular area that occupy more than 90% of the tumor mass in the case of CCLs. In substance, the mixed-type CCA is mainly composed of neoplastic ductular proliferation consisting of small monotonous and/or anastomosing glands, strongly positive for K7 and K19, with tumour boundary characterized by HCC-like trabecular area and, in some cases, small areas of adenocarcinoma with scarce MUC production. Recent insights are also emerging with regard to pre-neoplastic lesions such the intraductal papillary neoplasm of the bile duct (IPNB). This is a newly described microscopic entity characterized by intraluminal papillary tumor(s) with MUC secretion (34). IPNB is a recognized precursor of invasive MUC-producing CCA. It accounts for approximately 10% of all resectable cases. They occur throughout the biliary tract, share some histologic and clinical features with IPMNs of the pancreas, and may underlie a carcinogenetic pathway different from that of conventional bile duct carcinomas arising from flat dysplasia. Given the high risk of harboring invasive carcinoma, they should be treated with complete resection (34,35). All types of CCAs are associated with rapid proliferation of tumor-associated stromal cells, which contributes to desmoplastic nature of this cancer. Cancer-associated fibroblasts are key players in CCA invasiveness and in the generation of a desmoplastic reaction in CCA. Stromal cells isolated from surgical resections of CCA have been recently characterized, being vimentin/α-SMA-positive and CK7/CK19-negative (36). Different theories have been considered to explain the involvement of stromal cells in tumour pathogenesis, such as, an epithelial-mesenchymal cross-talk, an epithelia to mesenchyma transition, and finally, the mutations of the stromal cells per se. Recently, pure and stable primary cultures of human bile duct epithelial cells and stromal cells from CCA surgical specimens have been realized and this could represent an useful tool to investigate CCA tumor-stroma interactions. Cancer invasiveness and metastasis require that tightly adherent epithelial cells are converted to a more motile phenotype expressing several mesenchymal features. During this process, some molecular programs typical of the mesenchymal phenotype are activated, such as S100A4, a member of the S100 family of small calcium-binding proteins, expressed by mesenchymal cells, macrophages, and by epithelial cells in mesenchymal transition (EMT). Recently, Fabris L. et al. (37) showed that nuclear S100A4 identifies a subset of CCA patients with a poor prognosis after surgical resection and, that nuclear expression of S100A4 increases CCA cells invasiveness and metastasis indicating S100A4 as potential therapeutic target (37). Interestingly, a study in mammalian neoplasia suggests that genetic alterations in the stromal cells may precede genotypic changes in the epithelial cells, recapitulating the role of normal mesenchyma in normal mammary duct development (38). This newly described mechanism could be also involved in CCA pathogenesis. The classification based on microscopic features, however, has currently scarce practical implications since no correlation exists with modalities of growth, clinical patterns or response to treatment. On the other hands, the vast majority of CCAs belong to two different microscopic forms that are the pure MUC-secreting from and the mixed type (see below) and, this minimizes the relevance of a microscopic classification.
CCA classification based on cell of origin
Many years of investigations and daily clinical practice suggest an alternative model of carcinogenesis where only a subset of cancer stem cells (CSCs) has the ability to proliferate extensively and form the tumor mass (12,39-44). Signaling pathways associated with oncogenesis, including the Notch, Sonic hedgehog and Wnt signaling play a major role in regulating stem cell self-renewal. Although the terms CSC and “cell-of-origin” have been used interchangeably, they are distinct concepts referring to cancer-initiating cells and cancer-propagating cells, respectively (12,39-44). The term “cell-of-origin” defines the normal cell that acquires the first cancer-promoting mutation(s); on the other side, the definition “CSC” indicates the cellular subset within the tumor that uniquely sustains malignant growth (12,39-44). However, an unresolved question regarding liver cancers is which cell has to be considered as cell of origin. Recently, detailed immunohistochemical studies revealed that a whole range of phenotypical traits of hepatocytes, cholangiocytes and progenitor cells can be seen in liver primitive tumors [hepatocarcinoma (HCC) and CCA] being consistent with an origin from the hepatic stem cell compartment within canals of Hering and the biliary tree stem cells located within peribiliary glands (PBGs) (25). Recent histological and molecular characterization of CCAs highlights the heterogeneity of this cancer that may emerge at different sites of the biliary tree and with different macroscopic or morphological features. Furthermore, different stem cell niches have been recently described in the liver and biliary tree suggesting this as the basis of the heterogeneity of IH- and EH-CCAs (14-21). The complexity of the organization of the liver stem cell compartments could underlie the CCA clinical-pathological heterogeneity and the criticisms in classifying primitive liver tumours. These recent advances highlight a possible new classification of CCAs based on cells of origin and this responds to the need of generating homogenous diagnostic, prognostic and, hopefully, therapeutic categories of IH- and EH-CCAs (25,45). CCA may arise from the epithelium lining bile ducts, from canal of Herings or from PBGs of the IH and EH biliary tree (14-21). The accurate comparison of lineage markers between normal and neoplastic cells can lead to individuate the cell-of-origin in different CCA subtypes (25). Recent studies, taking into consideration morphology, immunohistochemistry and molecular comparison with the cells of origin, demonstrated how IH-CCA is constituted by two different forms (20). A first form (MUC IH-CCA), supposed to originate from large intrahepatic bile ducts, is constituted by pure-MUC secreting adenocarcinoma and displays large similarities (pathology, molecular) with EH-CCA. A second form (mixed-IHCCA) that is supposed to originate from small intrahepatic bile ductules, is composed by areas of focal hepatocytic differentiation, areas of neoplastic ductular reaction and areas of MUC-secreting adenocarcinoma (46).
Nakanuma et al. (21) recently proposed a new classification of CCA taking into consideration the heterogeneity of progenitor/stem cells within the liver and the pathological similarities between biliary and pancreatic neoplasms. IH-CCA is usually classified into peripheral and hilar types grossly and histologically into adenocarcinoma and rare variants (21). The Authors classified IH-CCA into: (I) bile ductular type or cholangiolocarcinoma (CLC); (II) intraductal neoplasm type; (III) conventional (bile duct) type and; (IV) rare variants (21). CLC is thought to originate from canals of Hering/bile ductules where hHpSCs are located. Komuta et al. (46) showed that this subtype of CCA is mainly composed of CLC areas showing small monotonous and/or anastomosing glands, strongly positive for K7 and K19, with tumour boundary being characterized by HCC-like trabecular area and with some cases expressing CCA areas with scarce MUC production. Comparison of CLC with K19-positive HCC and with combined HCC-CCAs indicated a high homology (46,47). The clear origin of CCL from hHpSCs (46,47) or immediate descendent cells reserves high attention and the accurate histological observation searching for transitional zones within the tumour and the phenotype characterization are strongly recommended for a proper diagnosis. Following the maturation arrest theory one could speculate that CCL represent the result of a carcinogenetic process involving cells within the lineage derived from the hHpSCs and that the differentiation grade of the tumour reflects the grade of maturation of the cells primarily involved in the carcinogenesis process (Figure 1). In this view the CCAs arising from the interlobular bile duct (small bile duct type CCAs) could be considered a tumour arising from differentiated cells belonging to the hHpSC-derived lineage (Figure 1). The evidence that IH-CCA and EH-CCA may be dissimilar tumours is supported by the recent discovery that, in vitro, they express diverse cellular proteins and have different cellular shape, doubling time, chromosome karyotype and chemosensitivity (48). Similarly, researchers from France showed that hilar CCA express higher levels of MUC5AC (60% vs. 22%), Akt2 (64% vs. 36%), K8 (98% vs. 82%), annexin (56% vs. 44%) and less vascular epithelial growth factor (VEGF) (22% vs. 78%) in comparison to IH-CCA (49). Moreover, prognostic markers resulted differentially expressed, as hilar CCAs carried out stronger perineural invasion (83% vs. 42%) than peripheral CCAs (49). The different biological and molecular features strongly support the concept that IH-CCA and EH-CCA arise from different carcinogenetic processes and different cells-of-origin. Particularly relevant in the view of future clinical trials is the lower expression of VEGF in EH-CCA with respect to the IH-CCA, which could affect the response to anti-angiogenic based therapy. Relevant advantages in the way to a physiopathological classification of the CCAs has been recently achieved by Roskams et al. (20), which carried out a study aimed to investigate the CCA histological diversity in relation to the heterogeneity of cholangiocytes lining the biliary tree: hilar MUC producing cells versus peripheral cuboidal ductular cells or hHpSCs. They investigated the clinical-pathological and molecular features of 79 resected CCAs and their relationship with hHpSCs and, compared the spectrum of CCAs with respect to K19-positive or negative HCCs. According to this study, 52% of the CCAs were pure MUC producing whereas 48% showed mixed differentiation features including focal hepatocytic differentiation and CCL features. CCAs with mixed features (mixed-CCAs) showed peripheral location, larger tumour size, less microvascular invasion, less lymph node involvement compared to pure MUC producing CCAs which showed hilar location, smaller tumour size, more microvascular invasion and more lymph node involvement. S100p expression was seen only in CCAs, while NCAM expression was only present in mixed-CCAs and particularly in CLC. Molecular profiling showed high homology between mixed-CCAs and K19-positive HCCs (considered of hHpSCs origin). The authors concluded that mixed-CCAs and K19-positive HCCs have a similar molecular profile as the most peripheral ductules, containing hHpSCs, while MUC producing CCAs have a similar profile to MUC producing large IH and EH bile ducts, possibly reflecting the different cells-of-origin (20,25). Differences in clinical-pathological features between CCAs arising from small (interlobular bile ducts) or medium-large IH bile ducts are under investigations. Responding to the need of classifying IH-CCA in relation to the heterogeneity of the small vs. the medium-large IH bile ducts, recently Nakanuma et al. (21) proposed to separately consider a small bile duct type (peripheral type) and a large bile duct type (perihilar type). The former is mainly described as a tubular or micropapillary adenocarcinoma while the latter involves the IH large bile ducts. In accordance with phenotypic differences between interlobular and medium-large bile ducts, Aishima et al. (50) investigated 87 cases of IH-CCA smaller than 5 cm in diameter. They considered a hilar type IH-CCA, showing IH large bile duct involvement within the tumour, and a peripheral type contained preserved architecture of the portal triad. They demonstrated that the frequency of perineural invasion, lymph node metastasis, vascular invasion, IH metastasis and EH recurrence of IH-CCA from large ducts was significantly higher than that of IH-CCA from small ducts (50). The survival of patients with IH-CCA from large ducts was worse than that of patients with IH-CCA from small ducts (50). In our hypothesis the clinical-pathological differences observed among CCAs arising from small bile ducts and large bile ducts reflect the different lineage of origin, with the former arising from cells of the hHpSC-derived lineage and the latter arising from BTSC-derived lineage (Figure 1) (25). Also, the multiple lineages of origin could determine differences in signalling pathways or epigenetic mechanisms associated with the early phase of tumour development in the course of the hepatic and biliary diseases. By considering the process of maturation from the two different stem cell niches (canals of Hering and PBGs), one could expect that some IH-CCAs originate from cells within the lineage starting in the canals of Hering (hHpSC-derived lineage) while, other IH-CCAs and the EH-CCAs could originate from cells within the lineage starting in the PBGs (BTSC-derived lineage) of the medium-large IH and EH bile ducts (Figure 1). The former could be constituted, on the basis of the grade of maturation of the cell-of-origin (maturation arrest), by combined HCC-CCA, CCL and CCA of the small bile ducts (interlobular), while the latter by CCA of the large bile ducts with variable degree of MUC production (Figure 1) The relevance of a classification of CCA according the cells of origin is sustained also by emerging data concerning differential pathological and radiological findings in CCAs arising from different cells within the biliary tree. Indeed, hilar CCAs and MUC IH-CCAs showed specifically secondary cholangitis associated with parenchymal necrosis/inflammation, moreover, lymphatic and perineural invasion and stroma amount were significantly higher in hilar CCAs and MUC IH-CCAs compared with mixed IH-CCAs and CLCs. Hilar CCAs and MUC IH-CCAs showed similar histopathological aspects, whereas CLCs and mixed IH-CCAs were histologically overlapping. At dynamic contrast-enhanced imaging, all MUC IH-CCAs showed concentric filling at venous phase, whereas mixed IH-CCAs/CLCs showed washout in various patterns. These clinical-pathological emerging findings clearly all IH and EH MUC-producing CCA whereas distinguish the mixed-type CCA which on the contrary share similarity with CLC, combined HCC-CCA and CK19 positive HCC. This distinction reflects the different cells of origin of MUC producing CCA and mixed type CCA.
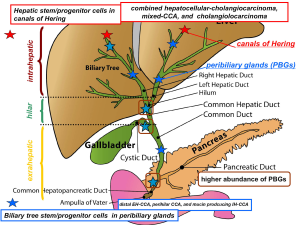
Comparison of the phenotype between Hepatic Stem Cells (hHpSCs) in Canals of Hering and Biliary Tree Stem/ Progenitor Cells (BTSCs) in PBGs, showed how BTSCs express markers of pluripotent stem cells (Nanog, OCT4), of definitive endoderm (LGR5, CXCR4, FoxA2) and of pancreatic progenitors (PDX1, NGN3), while hHpSCs is negative for early endodermic markers (39). The accurate comparison of lineage markers between normal and neoplastic cells can lead to individuate the cell of origin in different tumor subtypes arising within a given organ. Thus a morphological study comparing the candidate cells of origin with the respective CCA subtypes could elucidate the specific markers enable a classification of CCA based on the specific markers of the cell of origin. However, tumor cells show phenotypic plasticity or dedifferentiate during neoplastic progression, then lineage markers and molecular signatures of tumor cells may not precisely reflect the true cell of origin in normal tissue.
Conclusions
Recent advances highlighted large differences in clinical-pathological features of IH-CCAs arising from columnar MUC-producing cholangiocytes lining large bile ducts (MUC-CCA) versus IH-CCAs arising from cuboidal non MUC-producing cholangiocytes lining small bile ducts or from canal of Herings (mixed-CCAs). These recent results are opening a completely new scenario and break many paradigms in the field of primitive liver cancers. Indeed, the large bile duct MUC-producing IH-CCA has similarities with EH-CCA. In contrast, the small bile duct type (peripheral) or mixed type IH-CCA has features in common with ductular type cholangiolocellular carcinoma and with CK19+ HCC [97]. The clinical implications of these recent advances in terms of diagnostic tools, targeted therapy and indications for surgery or transplantation need accurate evaluations in the next future. In substance, the existence of two different stem cell compartments and the associated cell lineages may result in multiple cells of origin of CCA and could represent the basis of the clinico-pathological, epidemiological, and molecular heterogeneity of CCAs. These recent advances concerning the relationship between CCA types and normal stem cell counterparts, enable a CCAs classification based on cells of origin (25,45). Based on the grade of maturation of the cell of origin within the two lineages, CCAs can be reclassified as:
- CCAs originating from hHpSC-derived lineages that comprise combined hepatocellular-CCA, mixed-CCA, and CCL.
- Pure MUC-producing CCAs originating from hBTSC-derived lineages in PBGs or from epithelium of intra- or extrahepatic large bile ducts that comprise perihilar CCA and muc-IH-CCA.
A CCAs classification based on the cell-lineages-of-origin is more coherent with current knowledge on the epidemiology and risk factors and may have important clinical implications for the definition of specific therapeutic targets (25,45).
Challenges and needs
- Consistent nomenclature and clinical classification;
- Correct epidemiologic profile and risk factors;
- Macroscopic and microscopic subclassifications correlated with biology and therapeutic response;
- Appropriate markers (immunohistochemistry, molecular) or imaging techniques differentiating CCA subtypes.
Acknowledgements
D. Alvaro was supported by FIRB grant # RBAP10Z7FS_004 and by PRIN grant # 2009X84L84_002. V. Cardinale was supported by FIRB grant # RBAP10Z7FS_004. E. Gaudio was supported by research project grant from the University “Sapienza” of Rome and FIRB grant # RBAP10Z7FS_001 and by PRIN grant # 2009X84L84_001. The study was also supported by Consorzio Interuniversitario Trapianti d’Organo, Rome, Italy.
The authors thank Professor Lola McAdams Reid, Dr. Yunfang Wang, for the determinant contribution provided to achieve the original findings mentioned in this paper; Dr. Gemma Mendel for contribution in figure making.
Disclosure: The authors declare no conflict of interest.
References
- Callea F, Sergi C, Fabbretti G, et al. Precancerous lesions of the biliary tree. J Surg Oncol Suppl 1993;3:131-3. [PubMed]
- Nakanuma Y, Minato H, Kida T, et al. Pathology of cholangiocellular carcinoma. In: Tobe T, Kameda H, Okudaira M, et al. eds. Primary liver cancer in Japan. Tokyo, Japan: Springer-Verlag, 1994:39-50.
- Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med 1965;38:241-56. [PubMed]
- Nakanuma Y, Leong AS, Sripa B, et al. Intragepatic cholangiocarcinoma. In: Hamilton SR, Aaltonen LA. eds. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. Lyon: IARC Press, 2000.
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 2006;25:3818-22. [PubMed]
- Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873-5. [PubMed]
- Alvaro D, Crocetti E, Ferretti S, et al. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis 2010;42:490-5. [PubMed]
- Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol 2010;2:407-16. [PubMed]
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22. [PubMed]
- Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7.
- Fritz A, Percy C, Jack A, et al. eds. International classification of diseases for oncology (ICD-O). 3rd ed. Geneva, Switzerland: World Health Organization, 2000.
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [PubMed]
- Oishi N, Wang XW. Novel therapeutic strategies for targeting liver cancer stem cells. Int J Biol Sci 2011;7:517-35. [PubMed]
- Theise ND, Saxena R, Portmann BC, et al. The canals of Hering and hepatic stem cells in humans. Hepatology 1999;30:1425-33. [PubMed]
- Kuwahara R, Kofman AV, Landis CS, et al. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology 2008;47:1994-2002. [PubMed]
- Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med 2007;204:1973-87. [PubMed]
- Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011;54:2159-72. [PubMed]
- Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 2012;220:186-99. [PubMed]
- Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-45. [PubMed]
- Komuta M, Govaere O, Vander Borght S, et al. Histological diversity in cholangiocellular carcinoma suggesting different cells of origin: intrahepatic progenitor cells versus hilar mucin producing cells. J Hepatol 2011;54:S37.
- Nakanuma Y, Sato Y, Harada K, et al. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419-27. [PubMed]
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev 2005;1:253-60. [PubMed]
- Alison MR. Liver cancer: a disease of stem cells? Panminerva Med 2006;48:165-74. [PubMed]
- Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol 2009;217:282-98. [PubMed]
- Cardinale V, Carpino G, Reid L, et al. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol 2012;4:94-102. [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [PubMed]
- Wood R, Brewster DH, Fraser LA, et al. Do increases in mortality from intrahepatic cholangiocarcinoma reflect a genuine increase in risk? Insights from cancer registry data in Scotland. Eur J Cancer 2003;39:2087-92. [PubMed]
- Alvaro D, Crocetti E, Ferretti S, et al. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis 2010;42:490-5. [PubMed]
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198-203. [PubMed]
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657-69. [PubMed]
- Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 2nd English edition. Tokyo: Kanehara, 2003.
- Alvaro D, Bragazzi MC, Benedetti A, et al. Cholangiocarcinoma in Italy: A national survey on clinical characteristics, diagnostic modalities and treatment. Results from the “Cholangiocarcinoma” committee of the Italian Association for the Study of Liver disease. Dig Liver Dis 2011;43:60-5. [PubMed]
- Okuda K, Kubo Y, Okazaki N, et al. Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer 1977;39:232-46. [PubMed]
- Nakanuma Y, Sato Y. Cystic and papillary neoplasm involving peribiliary glands: a biliary counterpart of branch-type intraductal papillary mucinous [corrected] neoplasm? Hepatology 2012;55:2040-1. [PubMed]
- Rocha FG, Lee H, Katabi N, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012;56:1352-60. [PubMed]
- Massani M, Stecca T, Fabris L, et al. Isolation and characterization of biliary epithelial and stromal cells from resected human cholangiocarcinoma: a novel in vitro model to study tumor-stroma interactions. Oncol Rep 2013;30:1143-8. [PubMed]
- Fabris L, Cadamuro M, Moserle L, et al. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology 2011;54:890-9. [PubMed]
- Moinfar F, Man YG, Arnould L, et al. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res 2000;60:2562-6. [PubMed]
- Carpino G, Cardinale V, Reid L, et al. Cells of origin and cancer stem cells in cholangiocarcinoma. Transl Gastrointest Cancer 2012;1:33-43.
- Visvader JE. Cells of origin in cancer. Nature 2011;469:314-22. [PubMed]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68. [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [PubMed]
- Bailleul B, Surani MA, White S, et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell 1990;62:697-708. [PubMed]
- Brown K, Strathdee D, Bryson S, et al. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol 1998;8:516-24. [PubMed]
- Cardinale V, Carpino G, Reid LM, et al. Cholangiocarcinoma: a cancer in search of the right classification. Hepatology 2012;56:1585-6; author reply 1586. [PubMed]
- Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008;47:1544-56. [PubMed]
- Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 2006;12:410-6. [PubMed]
- He XR, Wu XP. Difference in biological characteristics and sensitivity to chemotherapy and radiotherapy between intrahepatic and extrahepatic cholangiocarcinoma cells in vitro. Chin Med Sci J 2008;23:54-9. [PubMed]
- Guedj N, Zhan Q, Perigny M, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol 2009;51:93-101. [PubMed]
- Aishima S, Kuroda Y, Nishihara Y, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol 2007;31:1059-67. [PubMed]


