
Modification of the 8th AJCC staging system of pancreatic ductal adenocarcinoma
Accurate cancer staging is critical not only for planning the clinical management, but also for predicting prognosis in patients with pancreatic ductal adenocarcinoma (PDAC). The former American Joint Committee on Cancer (AJCC) staging system (7th edition) for PDAC has failed to show prognostic relevance of T stage since vast majority (>90%) of patients who underwent pancreatectomy were classified as T3 (1,2). Moreover, the definition of T3 as tumor extension beyond pancreas without involvement of the celiac axis or superior mesenteric artery (SMA) gave rise to significant inter-observer/institutional variability due to lack of true capsule in the pancreas and highly variable distribution of adipose tissue surrounding the pancreatic parenchyma. To address these issues, the current AJCC staging system for PDAC (8th edition) uses only tumor size for T1 to T3 (T1, ≤2 cm; T2, >2 cm and ≤4 cm; T3, >4 cm), but maintains the T4 definition as tumor of any size involving the celiac axis, SMA and/or common hepatic artery. The current AJCC staging also subclassifies lymph node (LN) positive group into N1 (1–3 positive LNs) and N2 (≥4 positive LNs). The current 8th AJCC staging system allows for better reproducibility for T stage classification and better patient stratification compared to AJCC 7th edition (1).
To improve the 8th AJCC staging system for PDAC, Shi et al. performed in-depth analysis of two large cohorts of PDAC patients: 45,856 from the Surveillance, Epidemiology, and End Results (SEER) database [2004–2014] and 3,166 from Fudan University Shanghai Cancer Center (FUSCC) database [2005–2015]. The study investigated the impact of T stage on survival in patients with stage IIB (T1-3N1M0) and III (T1-3N2M0 or T4NanyM0) disease. They demonstrated that there were significant differences in overall survival (OS) among T1N1M0, T2N1M0 and T3N1M0 groups (median OS: 23.0, 19.0 and 16.0 months, respectively, P<0.001) and that T1N1M0 group had longer survival than that for stage IIA (T3N0M0) group (23.0 vs. 20.0 months; P<0.001). In addition, they also found T1N2M0 group had longer survival than that for stage IIB (T3N1M0) group (20.0 vs. 16.0 months; P<0.001). Furthermore, their study reported that the median OS of T3N2M0 group had longer OS than that of T4NanyM0 group in both SEER and FUSCC cohorts (P<0.001) (3). These results highlight the importance of T stage not only in LN negative PDAC patients, but also in those with N1 or N2 disease. Based on these findings, they proposed a modified TNM grouping system for the 8th AJCC staging system, which maintains the current T, N, and M definitions (Table 1) (3). In their study, the modified AJCC staging system has better concordance index for local disease than the 8th AJCC staging system (3). Thus, the modified AJCC staging system may better stratify PDAC patients for optimal management and prognosis, and should be considered for future revision of the AJCC staging system for PDAC.
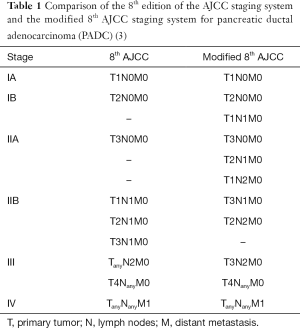
Full table
Number of examined LNs (ELNs) is critical for accurate N staging and has been shown to be one of the important prognostic factors in PDAC patients in previous studies and the study by Shi et al. (3-5). Shi et al. showed that patients with ≥15 ELNs were 48.4% and 46.2% in SEER and FUSCC cohorts, respectively. Given the importance of accurate N staging for PDAC, they performed similar survival analysis in patients with ≥15 ELNs from SEER and FUSCC cohorts. They confirmed the modified AJCC staging system was better in predicting survival in patients with ≥15 ELNs in both cohorts (3). Similar to previous studies, they found patients with ≥15 ELNs survived longer than those with <15 ELNs within the same substage group. These results clearly highlight the importance of examining adequate number of LNs in pancreatectomy specimens for accurate N staging (4,5). The 8th AJCC manual and the College of American Pathologists (CAP) protocol recommend that examination of a minimum of 12 ELNs is needed for accurate N staging. However, the minimum number of ELNs recommended by the International Study Group on Pancreatic Surgery (ISGPS) is 15. Standard guidelines and grossing protocols should be established to maximize the number of ELNs in pancreatectomy specimens.
It is important to note that the AJCC staging system for PDAC is based on patient populations who did not receive neoadjuvant therapy. Recently, neoadjuvant therapy has been commonly used to treat patients with potentially resectable PDAC, especially for those with borderline resectable PDAC. Post-treatment T and N stage based on either 7th or 8th AJCC staging system are important prognostic factors in PDAC patients who received neoadjuvant therapy (6,7). Chatterjee et al. showed that the 8th AJCC ypT stage better stratified patients based on survival than the 7th AJCC ypT stage (6). Although the 8th AJCC staging system is aimed to achieve better reproducibility in T staging, accurate tumor size measurement (ypT stage) is extremely challenging in pancreatectomy specimens after neoadjuvant therapy due to extensive therapy-induced fibrosis in both tumor and adjacent non-neoplastic pancreatic parenchyma. Therapy-induced fibrosis masks the boundary between tumor and adjacent benign pancreatic tissue and renders it difficult to accurately measure tumor size. The CAP protocol recommends gross measurement of tumor size be validated by histology. Therefore, systemic tumor mapping and generous sampling of the possible tumor area are needed to accurately measure tumor size based on gross and microscopic examination.
Another important issue is whether the same cut-off values for T stage (T1, ≤2 cm; T2, >2 cm and ≤4 cm; T3, >4 cm) should be used for both treatment-naïve and treated PDAC. Chatterjee et al. showed that patients with ypT1a and ypT1b had longer survival than those with ypT1c, but no significant difference in survival was found between ypT1c and ypT2 (6). Their study suggests tumor size of 1.0 cm is a better cut-off for ypT2 in PDAC patients who received neoadjuvant therapy. Future studies are needed to confirm their findings and to determine the optimal tumor size cut-off for ypT designation.
Acknowledgments
Funding: Supported by the National Institutes of Health grant (1R01 CA196941).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185-91.
- Saka B, Balci S, Basturk O, et al. Pancreatic ductal adenocarcinoma is spread to the peripancreatic soft tissue in the majority of resected cases, rendering the AJCC T-stage protocol (7th edition) inapplicable and insignificant: a size-based staging system (pT1: ≤2, pT2: >2–≤4, pT3: >4 cm) is more valid and clinically relevant. Ann Surg Oncol 2016;23:2010-8.
- Shi S, Hua J, Liang C, et al. Proposed modification of the 8th edition of the AJCC staging system for pancreatic ductal adenocarcinoma. Ann Surg 2019;269:944-50.
- Huebner M, Kendrick M, Reid-Lombardo KM, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:920-6. [Crossref] [PubMed]
- Vuarnesson H, Lupinacci RM, Semoun O, et al. Number of examined lymph nodes and nodal status assessment in pancreaticoduodenectomy for pancreatic adenocarcinoma. Eur J Surg Oncol 2013;39:1116-21. [Crossref] [PubMed]
- Chatterjee D, Katz MH, Foo WC, et al. Prognostic significance of new AJCC tumor stage in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant therapy. Am J Surg Pathol 2017;41:1097-104. [Crossref] [PubMed]
- Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:268-77. [Crossref] [PubMed]


