Network meta-analysis on prophylactic regimens against recurrent hepatitis B virus infection after liver transplantation
Introduction
Chronic hepatitis B virus (HBV) infection, affecting about 400 million people worldwide, is a leading cause of liver-related morbidity and mortality (1,2). It may cause hepatic cirrhosis, liver failure, and hepatocellular carcinoma (HCC) (3). Liver transplantation is the only option for those presenting with end-stage liver disease. However, recurrent HBV infection is common in liver graft recipients with chronic hepatitis B before the availability of immunoprophylaxis (Hepatitis B immunoglobulin, HBIG) and antiviral agents (lamivudine, LAM; adefovir, ADV) (4).
HBIG was firstly utilized to prevent post-transplant recurrence of hepatitis in 1978 (5). Previous study reported substitution of HBIG with LAM was effective for prevention of HBV recurrence in low-risk liver transplant recipients (6). Systematic review has already highlighted that combination treatment with HBIG and LAM reduced HBV recurrence following liver transplantation, compared with HBIG or LAM alone, whereas the included studies were all non-randomized (7). Latest clinical research showed combination of ADV with LAM could provide equivalent protection against recurrent HBV infection, compared with HBIG plus LAM prophylaxis (8). However, the relative effects of current regimens in prophylaxis against HBV recurrence based on irrefutably evidences of are still to be studied.
Network meta-analysis, also known as multiple-treatments comparison, enables us to synthesize data from both direct (within-trial comparisons) and indirect comparisons (inter-trial treatment comparisons through a common comparator treatment) of diverse regimens (9,10). We aimed to provide a clinically useful summary of the results from network meta-analysis that compared all the currently available treatments for preventing post-liver transplantation hepatitis B recurrence.
Materials and methods
Study searching
All relevant articles were retrieved from PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library using a combination of the terms “liver transplantation”, “Hepatitis B virus”, “HBV”, “hepatitis B immunoglobulin”, “HBIG” and “lamivudine”. An additional search through Google Scholar and a manual search through published literature were additionally performed. Two authors (ZY and KS) searched independently. There was no restriction of language or year.
Inclusion and exclusion criteria
Eligible studies included: (I) randomized controlled trials (RCTs) on prophylaxis against HBV recurrence after liver transplantation, studying patients with HBV-related disease; (II) prophylaxis with HBIG mono-therapy, antivirals, or both for prevention of recurrent HBV infection; (III) integrated description of methods and baseline characteristics; (IV) the trials giving definite criteria of HBV recurrence and recurrence rates; (V) allogeneic liver recipients were included, gender, age and nationality were not restricted.
The following exclusion criteria were used: (I) case series, case reports, reviews and conference reports; (II) studies lacking a control group; (III) studies that were unable to provide clear baseline characteristics; (IV) patients treated for other hepatitis virus related diseases, such as type C or D.
Outcomes
The outcomes measured were recurrence rates within the first year and the rates throughout the whole follow-up. Recurrence of HBV infection was denoted as reappearance of hepatitis B surface antigen (HBsAg) in the serum after transplantation. The recurrent events were counted from the randomization.
Data extraction and quality assessment
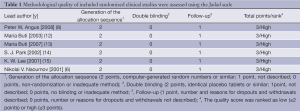
Two reviewers (FW and WX) independently selected the studies and extracted data following inclusion and exclusion criteria. Data discrepancies between the two reviewers were discussed by the two investigators to reach consensus. Authors of included studies were contacted when clarification was needed. Other two reviewers (ZY and KS) assessed the trials independently. The methodological quality of included randomized clinical studies was assessed using the Jadad scale (11), including generation of the allocation sequence, double blinding and follow-up (Table 1). All of the included studies were of high quality.
Full table
Statistical analysis
First, we conducted pair-wise meta-analyses with a random-effects model to synthesize studies comparing the same pair of treatments. The results were reported as pooled risk ratio (RR) with the corresponding 95% confidence interval (CI). Statistical heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). Statistical significance was regarded as P<0.05. All calculations were performed using REVIEW MANAGER (version 5.0 for Windows; the Cochrane Collaboration, Oxford, UK).
Second, we built a random-effects network within a Bayesian framework using Markov chain Monte Carlo methods in ADDIS 1.15 (Drugis.org) (16). We networked the translated binary outcomes of HBV recurrence rates within studies and specified the relations among the RRs across studies making different comparisons as previously reported (17), with which, direct and indirect evidences for any given pair of treatments were combined. We used P<0.05 and 95% CIs beyond the null value to assess significance.
We also estimated the probability of each treatment being as the best regimen, the second best, the third best and so on, by calculating the RR of each treatment group compared with arbitrary common controls, and counting the proportion of iterations of the Markov chain of the RR ranking in treatments. We ranked treatments in terms of the risk of HBV recurrence with the same methods.
A variance calculation and a node-splitting analysis provided by the software ADDIS 1.15 were applied to evaluate the inconsistency within the network meta-analysis. Significant inconsistency existed if the difference between random effects variance and inconsistency variance was large or a P<0.05 of disagreement between direct and indirect evidences was met. We would adjust the study included and ultimately obtain an ideal network with consistency according to quantitative estimation.
Results
Eligible studies
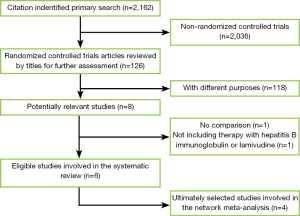
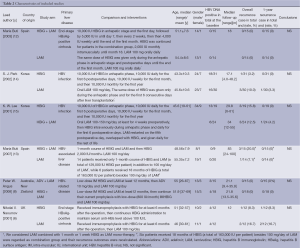
We identified 2,162 records according to the search strategy and finally included six RCTs that compared HBIG, LAM, ADV or their combinations in prophylaxis of HBV recurrence after liver transplantation for HBV related diseases (6,8,12-15). Figure 1 was flow chart. However, we excluded the studies by Naoumov et al. (6) and Angus et al. (8) due to long-term post-transplantation treatments of HBIG before randomization. A total of 162 patients from four ultimately selected RCTs were involved. There were 50, 58 and 54 patients allocated to HBIG mono-therapy, LAM mono-therapy and HBIG in combination with LAM, respectively. The range of median follow-up was 17.1 to 83 months among included studies. The enrollment of patients in Buti’s studies was basically with no overlap between 2007 and 2003 (12,13). Notably, all of our included RCTs didn’t show significant difference in pairs. Table 2 summarized the characteristics of all involved studies.
Full table
Direct meta-analysis
In terms of overall HBV recurrence rate after liver transplantation, we found combination of HBIG and LAM showed comparable efficacy of prevention with LAM mono-therapy by Buti’s studies with an RR of 2.00 (95% CI: 0.34 to 11.83; P=0.44) and included studies of HBV recurrence rate were homogeneous (Chi2 =0.43, P=0.51, I2 =0%) (12,13). The two groups represented similar outcomes in recurrence within one year after randomization (RR 0.93, 95% CI: 0.12 to 7.08; P=0.44; heterogeneity: Chi2 =0.00, P=1.00, I2 =0%).
Network meta-analyses for the risk of HBV recurrence
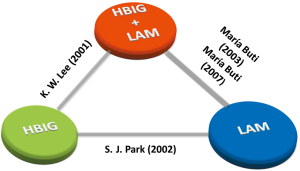
We established a network for comparisons of HBIG mono-therapy, LAM mono-therapy and combination therapy of HBIG and LAM (see Figure 2). Figure 3 summarized the results of the network meta-analyses regarding HBV recurrence rates. According to the results, HBIG mono-therapy, LAM mono-therapy and HBIG plus LAM showed no statistically difference in RRs in terms of overall HBV recurrence rates. Nevertheless, HBIG mono-therapy had potential advantage compared with combination of HBIG and LAM in terms of 1-year HBV recurrence rate (RR 0.00, 95% CI: 0.00 to 0.91). The rest comparisons revealed no significance in 1-year HBV recurrence rate.

Rank probabilities
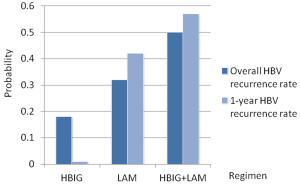
Figure 4 shows the treatment ranking of probability to be the worst treatment. Agents with greater value of the histogram were associated with greater probabilities for the highest HBV recurrence rate. Based on network, the cumulative probabilities of treatments being associated with the highest recurrence were (overall HBV recurrence rate, 1-year HBV recurrence rate): HBIG (18%, 1%), LAM (32%, 42%) and HBIG combined with LAM (50%, 57%). In other words, for both overall and 1-year recurrence rates, HBIG mono-therapy was the most likely to have the best prophylaxis efficacy, followed by LAM mono-therapy, combination of LAM and HBIG, sequentially.
Discussion
Previous meta-analyses indicated that the HBIG and LAM combination therapy was significantly better than single HBIG or LAM for preventing HBV recurrence after transplantation (7,18,19). However, all these meta-analyses were based on predominantly retrospective non-randomized studies in which patient selection bias existed. For instance, most of these retrospective studies used historical controls with poor comparability (18,19). The true relative effect of combination group versus mono-therapy might be confounded by other factors, which lead to artefact that combination of LAM and HBIG is superior to single agent administration. RCTs could minimize the confounding impact, implying the most objective comparison. The results from this purely RCTs based network meta-analysis were inconsistent with previous views. Our results showed no significant differences in overall HBV recurrence rate after liver transplantation among HBIG mono-therapy, LAM mono-therapy, HBIG combined with LAM, but revealed decreasing potential advantage sequentially. Besides, we found similar results between all the regimens in terms of 1-year HBV recurrence rate, and even significant benefits in HBIG mono-therapy compared with combination of HBIG and LAM. In addition, rank probabilities revealed incidences of HBV recurrence in HBIG mono-therapy, LAM mono-therapy and HBIG combined with LAM were ranged from low to high.
Mechanically, HBIG neutralizes circulating virus particles and induces lysis of infected hepatocytes (20), while antivirals directly reduce viral load at intrahepatic and extrahepatic sites (21). Previous studies showed decreasing virions by HBIG reduced the viral substrate for antivirals, and reduced the occurrence of drug-resistant mutants (22). While lowering viral load caused by antivirals might inhibit the saturation of HBIG binding sites and decrease the immune pressure, leading to emergence of surface antigen mutations (7). Unfortunately, mutations in the surface gene may cause changes in the polymerase gene and vice versa because of overlapping reading frames in HBV genome. As a result, the use of combination treatment may lead to selection of mutations that reduce the efficacy of each of the drugs, therefore causing HBV recurrence (7). Based on our results and the evidences above, we thought HBIG mono-therapy might be critical for early treatment instead of combination therapy in prophylaxis of HBV recurrence after liver transplantation. LAM might be more sensitive if viral load was heavier and should be chosen as a replacement therapy when HBIG was not effective. Untimely combination of HBIG and LAM might be prone to drug resistance. In addition, as intravenous injection of HBIG is more expensive and less convenient than LAM, inflexible usage of combination therapy can be less cost-effective (23). Whether combination therapy should be the first choice is debatable. Which therapeutic regimen should be chosen timely that can show superiority in preventing HBV recurrence after liver transplantation is still worthy of further research.
This is the first multiple-treatment comparison for the currently available methods in prophylaxis of post-liver transplantation HBV recurrence based on evidences with good quality, which argued with the present consensus of combining LAM and HBIG. However, there existed several limitations. First, the number and sample size of the included studies were still inadequate to draw substantial conclusion. Second, different lengths of median follow-up among the included trials might lead to heterogeneity of outcomes. Third, we failed to evaluate some important clinical outcomes including patient survival and adverse reaction of agents in the current study since these data were reported by few included trials. Therefore, future large sample sized RCTs which would optimize the network and multiple-treatment comparison based on detailed clinical outcomes are warranted to further clarify our assumption. Novel anti-HBV agents such as entecavir, telbivudine or tenofovir were expected to be included.
In conclusion, this network meta-analysis showed no significant differences among HBIG mono-therapy, LAM mono-therapy, combination of HBIG and LAM in overall HBV recurrence rate after liver transplantation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol 2003;39:S64-9. [PubMed]
- Nair SN, Perrillo RP. Hepatitis B and D. In: ZakimD, Boyer TD. eds. Hepatology: A Textbook of Liver Disease. 4th ed. Philadelphia: Saunders, 2003;2:959-1016.
- Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet 2003;362:2089-94. [PubMed]
- Eason JD, Freeman RB Jr, Rohrer RJ, et al. Should liver transplantation be performed for patients with hepatitis B? Transplantation 1994;57:1588-93. [PubMed]
- Johnson PJ, Wansbrough-Jones MH, Portmann B, et al. Familial HBsAg-positive hepatoma: treatment with orthotopic liver transplantation and specific immunoglobulin. Br Med J 1978;1:216. [PubMed]
- Naoumov NV, Lopes AR, Burra P, et al. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol 2001;34:888-94. [PubMed]
- Katz LH, Paul M, Guy DG, et al. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis 2010;12:292-308. [PubMed]
- Angus PW, Patterson SJ, Strasser SI, et al. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology 2008;48:1460-6. [PubMed]
- Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. [PubMed]
- Ades AE, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1-19. [PubMed]
- Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [PubMed]
- Buti M, Mas A, Prieto M, et al. A randomized study comparing lamivudine monotherapy after a short course of hepatitis B immune globulin (HBIg) and lamivudine with long-term lamivudine plus HBIg in the prevention of hepatitis B virus recurrence after liver transplantation. J Hepatol 2003;38:811-7. [PubMed]
- Buti M, Mas A, Prieto M, et al. Adherence to lamivudine after an early withdrawal of hepatitis B immune globulin plays an important role in the long-term prevention of hepatitis B virus recurrence. Transplantation 2007;84:650-4. [PubMed]
- Park SJ, Paik SW, Choi MS, et al. Is lamivudine with 1-week HBlg as effective as long-term high-dose HBlg in HBV prophylaxis after liver transplantation? Transplant Proc 2002;34:1252-4. [PubMed]
- Lee KW, Lee SK, Joh JW, et al. Comparison of the efficacy in prevention of hepatitis B virus recurrence after liver transplantation between HBIG and lamivudine. Transplant Proc 2001;33:3643-4. [PubMed]
- Van Valkenhoef G, Tervonen T, Zwinkels T, et al. ADDIS: a decision support system for evidence-based medicine. Decision Support Systems 2012;55:459-75.
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009;373:746-58. [PubMed]
- Loomba R, Rowley AK, Wesley R, et al. Hepatitis B immunoglobulin and Lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol 2008;6:696-700. [PubMed]
- Rao W, Wu X, Xiu D. Lamivudine or lamivudine combined with hepatitis B immunoglobulin in prophylaxis of hepatitis B recurrence after liver transplantation: a meta-analysis. Transpl Int 2009;22:387-94. [PubMed]
- Yi NJ, Suh KS, Cho JY, et al. Recurrence of hepatitis B is associated with cumulative corticosteroid dose and chemotherapy against hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2007;13:451-8. [PubMed]
- Schreibman IR, Schiff ER. Prevention and treatment of recurrent Hepatitis B after liver transplantation: the current role of nucleoside and nucleotide analogues. Ann Clin Microbiol Antimicrob 2006;5:8. [PubMed]
- Han SH, Ofman J, Holt C, et al. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl 2000;6:741-8. [PubMed]
- Zoulim F. Towards an improved and cost-saving prophylaxis of hepatitis B virus recurrence after liver transplantation? J Hepatol 2003;38:850-2. [PubMed]