Prognostic significance of TP53 expression for patients with hepatocellular carcinoma: a meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of death from cancer and the third largest cause of cancer-related deaths worldwide (1). Although liver resection is an effective treatment for patients with advanced HCC, the long-term postoperative prognosis remains poor because of the high recurrence rate and lack of effective systemic therapy for HCC patients with metastases. The main prognostic factors are clinicopathological characteristics of the disease, including tumor size, stage, and grade. However, the prognostic factors do not fully predict individual clinical outcome. There is the need for better markers to identify patients with poor prognosis at the time of diagnosis. Studies have focused on the potential role of new biological factors involved in the carcinogenic process as prognostic markers in patients with HCC.
Tumor suppressor gene p53, its wild-type protein is responsible for cell-cycle regulation and apoptosis after DNA damage. If p53 is mutated, however, the cell with DNA damage can escape from apoptosis and turn into cancer cells (2). Furthermore, the mutant p53 protein, which lost the function of wild-type protein, can accumulate in cell nuclei and is regarded as a highly specific indicator of malignancy (3). To date, some studies have documented that p53 alterations are correlated with tumor differentiation, vascular invasion, tumor stage, Child-Pugh class and serum AFP in HCC (4-7).
Many studies have evaluated whether p53 expression may be a prognostic factor for survival in patients with HCC. However, the results of the studies are inconclusive and no consensus has been reached. It is unknown whether differences in these investigations have been mostly due to their limited sample size or genuine heterogeneity. Thus, we conducted a meta-analysis of all available studies relating p53 expression with the clinical outcome in patients with HCC.
Materials and methods
Search strategy and study selection
The electronic databases PubMed was searched for studies to include in the present meta-analysis. An upper date limit of Dec 30, 2013 was applied; we used no lower date limit. Searches included the terms “hepatocellular or liver”, “cancer or carcinoma or tumor or neoplasm”, “p53”, “expression” and “prognosis”. We also reviewed the Cochrane Library for relevant articles. The references reported in the identified studies were also used to complete the search.
Studies eligible for inclusion in this meta-analysis met the following criteria: (I) measure p53 expression in the primary HCC; (II) provide information on survival [i.e., disease free survival (DFS) and/or overall survival (OS), studies investigating response rates only were excluded] and (III) when the same author reported results obtained from the same patient population in more than one publication, only the most recent report, or the most complete one, was included in the analysis. Two reviewers (P.Z. and Y.J.) independently determined study eligibility. Disagreements were resolved by consensus.
Data extraction and quality assessment
Data retrieved from the reports included author, publication year, patient source, study design, test method, p53 expression positive ratio and survival data (Table 1). If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”. We did no contact the author of the primary study to request the information. We did not use prespecified quality-related inclusion or exclusion criteria and did not weigh each study by a quality score, because the quality score has not received general agreement for use in a meta-analysis, especially observational studies (32). The data extraction and quality assessment could refer to our previous published meta-analysis (33-36).
Statistical methods
Included studies were divided into two groups for analysis: those with data regarding OS and those regarding DFS. For the quantitative aggregation of the survival results, we measured the impact of p53 expression on survival by hazard ratio (HR) between the two survival distributions. HRs and 95% confidence intervals (CIs) were used to combine as the effective value. If the HRs and their 95% CIs were given explicitly in the articles, we used crude ones. When these variables were not given explicitly, they were calculated from the available numerical data using methods reported by Parmar et al. (37).
Heterogeneity of the individual HRs was calculated with χ2 tests according to Yusuf et al.’s method (38). Heterogeneity test with inconsistency index (Ι2) statistic and Q statistic was performed. If HRs were found to have fine homogeneity, a fixed effect model was used for secondary analysis; if not, a random-effect model was used. DerSimonian-Laird random effects analysis (39) was used to estimate the effect of p53 mutation on survival. By convention, an observed HR >1 implies worse survival for the group with p53 mutation. The impact of p53 mutation on survival was considered to be statistically significant if the 95% CI did not overlap with 1. Horizontal lines represent 95% CIs. Each box represents the HR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (HR =1.0).
Evidence of publication bias was sought using the methods of Egger et al. (40) and of Begg et al. (41). Intercept significance was determined by the t-test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias). All of the calculations were performed by STATA version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study selection and characteristics
Twenty-four studies (8-31) published between 1996 and 2012 were eligible for this meta-analysis. All reported the prognostic value of p53 expression status for survival in HCC patients. The total number of patients included was 2,585, ranging from 20 to 400 patients per study (median 107). The major characteristics of the 24 eligible publications are reported in Table 1.
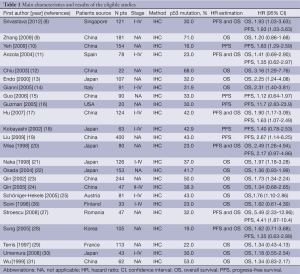
Full table
All of the studies reported the prognostic value of p53 expression status for survival in patients with HCC tissue. Of the 24 studies, 18 directly reported HRs (multivariate analysis), while the other six studies provided survival curves. Among them, the proportion of patients exhibiting p53 overexpression in individual studies ranged from 16% to 43%. Estimation using survival curves were segregated according to either OS or DFS. A HR on DFS and OS could be extracted for 11 publications and 19 publications of studies, respectively.
Meta-analysis
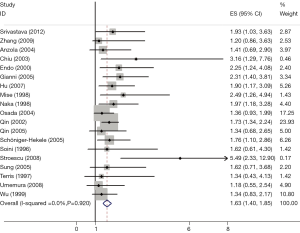
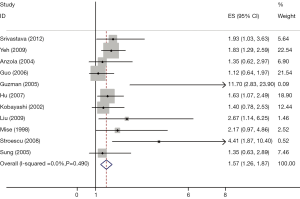
The results of the meta-analysis were shown in Table 2 and Figures 1,2. Overall, the combined HR for all 19 eligible studies evaluating p53 overexpression on OS was 1.64 (95% CI: 1.40-1.85), suggesting that p53 overexpression was an indicator of poor prognosis for HCC. No significant heterogeneity was observed among the studies (Q=2.24, I2=0.0%, P=0.920). Meanwhile, for DFS analysis including 11 studies, statistically significant effect of p53 overexpression (HR =1.57, 95% CI: 1.26-1.87) in patients with HCC was also observed (Q=5.27, I2=0.0%, P=0.490).

Full table
Publication bias
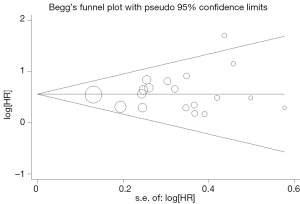
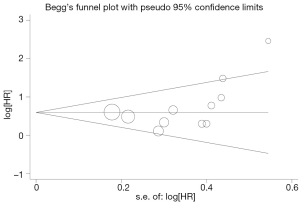
Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literature. All 19 eligible studies investigating p53 overexpression on OS yielded a Begg’s test score of P=0.105 and an Egger’s test score of P=0.262, meanwhile according to the funnel plot (Figure 3), the absence of publication bias was found. For DFS analysis, no publication biases were found for investigating p53 overexpression of 11 studies [a Begg’s test score of P=0.208 and an Egger’s test score of P=0.148 (Figure 4)].
Discussion
HCC has poor prognosis and high recurrence rate, regardless of the treatment. Therefore, it is imperative for clinicians and scientists to find new ways to stratify patients for appropriate treatment. Previous reports have attempted to build a model based on the prognostic value of putative hepatic stem cell biomarkers in HCC (42). Traditionally, however, tumor staging system (TNM and BCLC staging), tumor size and serum AFP levels are used to predict the outcome of HCC patients, which sometimes cannot accurately predict the outcome of all HCC patients (43). Up to date, there is neither any molecular marker routinely incorporated to staging systems, nor there is a molecular prognostic model. The present meta-analysis has combined 24 publications including 2,585 patients to yield statistics, indicating a statistically significant role of p53 overexpression on OS and DFS in HCC.
Our data were consistent with the results of a previous meta-analysis (44) published in 2011 that showed an association between p53 aberration and poor survival of patients with HCC. We have improved upon that previous meta-analysis by including more recent related studies and by generally using a more comprehensive search strategy. Screening, study selection and quality assessment were performed independently and reproducibly by two reviewers. We also explored heterogeneity and potential publication bias in accordance with published guidelines.
The heterogeneity issue was complicated in the systematic review and meta-analysis was. We found no significant heterogeneity among all studies included and subgroup analysis. Another potential source of bias is related to the method of HR and 95% CI extrapolation. If these statistics were not reported by the authors, we calculated them from the data available in the article. If this was not possible, we extrapolated them from the survival curves, necessarily making assumptions about the censoring process. Data for multivariate survival analysis reported in the article were included in the present systematic review and meta-analysis; if these data were not available, data calculated from survival curves by univariate analysis were included. These results should be confirmed by an adequately designed prospective study. Furthermore, the exact value of p53 expression needs to be determined by appropriate multivariate analysis.
Publication bias (45) is a major concern for all forms of meta-analysis; positive results tend to be accepted by journals, while negative results are often rejected or not even submitted. The present analysis does not support publication bias; the obtained summary statistics likely approximate the actual average. However, it should be noted that our meta-analysis could not completely exclude biases. For example, the study was restricted to papers published in English and Chinese, which probably introduced bias.
In conclusion, our meta-analysis estimated the association between prognostic significance of p53 expression and patients with HCC. As determined in our meta-analysis, we concluded that p53 expression was associated with poor OS and DFS.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials 2008;3:31-9. [PubMed]
- Lai PB, Chi TY, Chen GG. Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepato-cellular carcinoma cell line in vitro. Apoptosis 2007;12:387-93. [PubMed]
- Dowell SP, Wilson PO, Derias NW, et al. Clinical utility of the immunocytochemical detection of p53 protein in cytological specimens. Cancer Res 1994;54:2914-8. [PubMed]
- Park NH, Chung YH, Youn KH, et al. Close correlation of p53 mutation to microvascular invasion in hepatocellular carcinoma. J Clin Gastroenterol 2001;33:397-401. [PubMed]
- Terris B, Laurent-Puig P, Belghitti J, et al. Prognostic influence of clinicopathologic features, DNA-ploidy, CD44H and p53 expres-sion in a large series of resected hepatocellular carcinoma in France. Int J Cancer 1997;74:614-9. [PubMed]
- Yuan RH, Jeng YM, Chen HL, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol 2006;209:549-58. [PubMed]
- Atta MM, el-Masry SA, Abdel-Hameed M, et al. Value of serum anti-p53 antibodies as a prognostic factor in Egyptian patients with hepatocellular carcinoma. Clin Biochem 2008;41:1131-9. [PubMed]
- Srivastava S, Wong KF, Ong CW, et al. A morpho-molecular prognostic model for hepatocellular carcinoma. Br J Cancer 2012;107:334-9. [PubMed]
- Zhang MF, Zhang ZY, Fu J, et al. Correlation between expression of p53, p21/WAF1, and MDM2 proteins and their prognostic significance in primary hepatocellular carcinoma. J Transl Med 2009;7:110. [PubMed]
- Yeh CT, Kuo CJ, Lai MW, et al. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. BMC Cancer 2009;9:324. [PubMed]
- Anzola M, Saiz A, Cuevas N, et al. High levels of p53 protein expression do not correlate with p53 mutations in hepatocellular carcinoma. J Viral Hepat 2004;11:502-10. [PubMed]
- Chiu CT, Yeh TS, Hsu JC, et al. Expression of Bcl-2 family modulated through p53-dependent pathway in human hepatocel-lular carcinoma. Dig Dis Sci 2003;48:670-6. [PubMed]
- Endo K, Ueda T, Ohta T, et al. Protein expression of MDM2 and its clinicopathological relationships in human hepatocellular carcinoma. Liver 2000;20:209-15. [PubMed]
- Gianni S, Cecchetto A, Altavilla G, et al. Tumour staging, morphology and p53 overexpression concur in predicting survival in hepatocellular carcinoma. J Intern Med 2005;257:367-73. [PubMed]
- Guo RP, Zhong C, Shi M, et al. Clinical value of apoptosis and angiogenesis factors in estimating the prognosis of hepatocellular carcinoma. J Cancer Res Clin Oncol 2006;132:547-55. [PubMed]
- Guzman G, Alagiozian-Angelova V, Layden-Almer JE, et al. p53, Ki-67, and serum alpha feto-protein as predictors of hepatocellu-lar carcinoma recurrence in liver transplant patients. Mod Pathol 2005;18:1498-503. [PubMed]
- Hu TH, Wang CC, Huang CC, et al. Down-regulation of tumour suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen index predict poor patient outcome of hepatocellular carcinoma after resection. Oncol Rep 2007;18:1417-26. [PubMed]
- Kobayashi T, Sugawara Y, Shi YZ, et al. Telomerase expression and p53 status in hepatocellular carcinoma. Am J Gastroenterol 2002;97:3166-71. [PubMed]
- Liu AW, Cai J, Zhao XL, et al. The clinicopathological significance of BUBR1 overexpression in hepatocellular carcinoma. J Clin Pathol 2009;62:1003-8. [PubMed]
- Mise K, Tashiro S, Yogita S, et al. Assessment of the biological malignancy of hepatocellular carcinoma: relationship to clinicopathological factors and prognosis. Clin Cancer Res 1998;4:1475-82. [PubMed]
- Naka T, Toyota N, Kaneko T, et al. Protein expression of p53, p21WAF1, and Rb as prognostic indicators in patients with surgically treated hepatocellular carcinoma. Anticancer Res 1998;18:555-64. [PubMed]
- Osada S, Saji S, Kuno T. Clinical significance of combination study of apoptotic factors and proliferating cell nuclear antigen in estimating the prognosis of hepatocellular carcinoma. J Surg Oncol 2004;85:48-54. [PubMed]
- Qin LX, Tang ZY, Ma ZC, et al. p53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol 2002;8:459-63. [PubMed]
- Qin HX, Nan KJ, Yang G, et al. Expression and clinical significance of TAp73a, p53, PCNA and apoptosis in hepatocellular carcinoma. World J Gastroenterol 2005;11:2709-13. [PubMed]
- Schöniger-Hekele M, Hänel S, Wrba F, et al. Hepatocellular carcinoma-survival and clinical characteristics in relation to various histologic molecular markers in Western patients. Liver Int 2005;25:62-9. [PubMed]
- Soini Y, Virkajärvi N, Lehto VP, et al. Hepatocellular carcinomas with a high proliferation index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br J Cancer 1996;73:1025-30. [PubMed]
- Stroescu C, Dragnea A, Ivanov B, et al. Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointestin Liver Dis 2008;17:411-7. [PubMed]
- Sung CO, Yoo BC, Koh KC, et al. Prognostic significance of p53 overexpression after hepatic resection of hepatocellular carcinoma. Korean J Gastroenterol 2005;45:425-30. [PubMed]
- Terris B, Laurent-Puig P, Belghitti J, et al. Prognostic influence of clinicopathologic features, DNA-ploidy, CD44H and p53 expression in a large series of resected hepatocellular carcinoma in France. Int J Cancer 1997;74:614-9. [PubMed]
- Umemura A, Itoh Y, Itoh K, et al. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology 2008;47:493-502. [PubMed]
- Wu PC, Lau VK, Fang JW, et al. Imbalance between cell proliferation and cellular DNA fragmentation in hepatocellular carcinoma. Liver 1999;19:444-51. [PubMed]
- Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8. [PubMed]
- Zhan P, Qian Q, Yu LK. Prognostic value of COX-2 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2013;5:40-7. [PubMed]
- Zhan P, Qian Q, Wan B, et al. Prognostic value of TTF-1 expression in patients with non-small cell lung cancer: a meta-analysis. Transl Cancer Res 2013;2:25-32.
- Zhan P, Wang Q, Qian Q, et al. Megestrol acetate in cancer patients with anorexia-cachexia syndrome: a meta-analysis. Transl Cancer Res 2013;2:74-9.
- Zhan P, Wang Q, Qian Q, et al. Risk of venous thromboembolism with the erythropoiesis-stimulating agents (ESAs) for the treatment of cancer-associated anemia: a meta-analysis of randomized control trials. Chin Clin Oncol 2012;1:19.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform metaanalyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [PubMed]
- Yusuf S, Peto R, Lewis J, et al. Blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010;59:953-62. [PubMed]
- Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol 2004;130:497-513. [PubMed]
- Liu J, Ma Q, Zhang M, et al. Alterations of TP53 are associated with a poor outcome for patients with hepatocellular carcinoma: evidence from a systematic review and meta-analysis. Eur J Cancer 2012;48:2328-38. [PubMed]
- Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Statist Soc A 1988;151:419-63.


