Inhibition of the liver expression of arylalkylamine N-acetyltransferase increases the expression of angiogenic factors in cholangiocytes
Introduction
The intrahepatic biliary ductal system is lined by simple epithelia, cholangiocytes of different size and functions (1-3). In addition to secrete water and electrolytes (4,5), cholangiocytes are the target cells in a number of human biliary disorders such as primary sclerosing cholangitis and primary biliary cirrhosis, diseases that are characterized by an imbalance between biliary growth and loss (2,6,7). Constitutively, normal rodent cholangiocytes have low proliferative activity (8,9), but proliferate or are damaged in response to experimental maneuverers including ligation of the extrahepatic bile duct ligated (BDL) and acute carbon tetrachloride administration (9).
A number of studies support the concept that biliary development and homeostasis is coordinately regulated by several neuroendocrine autocrine factors including vascular endothelial growth factor-A/C (VEGF-A/C), angiopoietins, serotonin, melatonin and sex hormones (10-17). In fact, we have previously shown that normal cholangiocytes express the mRNA for VEGF-A/C and secrete VEGF-A/C that are upregulated following BDL (14). We have also shown that VEGFA/C stimulates biliary growth of normal and BDL rats by autocrine/paracrine pathways (14). In support of this concept, we have previously demonstrated that after BDL, the peribiliary plexus (that secrete angiogenic factors such as VEGF) proliferate supporting the increased nutritional needs of the proliferated biliary epithelium (18). However, the proliferation of the peribiliary plexus only occurs after cholangiocytes proliferation (18), supporting the concept that VEGF stimulates biliary growth by an autocrine loop. Another study has shown that cholangiocytes generate a VEGF gradient that is crucial during the migratory stage, when it determines arterial vasculogenesis in their vicinity, whereas angiopoietin-1 signaling from hepatoblasts contributes to the remodeling of the hepatic artery necessary to meet the demands of the developing epithelium (12). VEGF and angiopoietin-1 have autocrine proliferative effect on cholangiocyte growth and paracrine effect on portal vasculature, thus promoting the growth of the cysts and their vascular supply (11).
Serotonin N-acetyltransferase (AANAT), is the key enzyme for the synthesis of melatonin (19), and it is expressed by the pineal gland as well as small intestine and in the liver mostly by cholangiocytes (20). We have recently shown that melatonin secretion inhibits biliary hyperplasia by interaction with type 1 (MT1) receptors (17).
We have recently demonstrated that inhibition of biliary AANAT expression (by administration of Vivo-Morpholino sequences of AANAT) induces an increase in biliary proliferation, ductal mass and increases SR, CFTR, and Cl–/HCO3– AE2 expression (21). We have also shown that in vitro overexpression of AANAT in cholangiocyte cell lines decreases the basal proliferative rate and the expression of SR, CFTR, and Cl–/HCO3– AE2 in these cells (21). Thus, based on these findings we propose to demonstrate that in vivo and in vitro modulation of AANAT biliary expression induces change in the expression of VEGF-A/C, two key trophic factors sustaining biliary proliferation (15,18,22).
Materials and methods
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise stated. The substrate for γ-glutamyltranspeptidase (γ-GT), N (γ-L-glutamyl)-4-methoxy-2-naphthylamide, was purchased from Polysciences (Warrington, PA, USA). The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA): (I) VEGF-A (JH121) a mouse monoclonal antibody recognizing full length VEGF-A of human origin; and (II) VEGF-C (H-190) a rabbit polyclonal antibody against amino acids 230-419 of VEGF-C of human origin. Commercially available ELISA kits for the measurement of VEGF-A/C levels were obtained from RayBiotech, Inc., Norcross, GA, USA.
The RNeasy Mini Kit for the isolation of total RNA was purchased from Qiagen (Valencia, CA, USA). The Vivo-Morpholino sequences of AANAT (5'-GTTCCCCAGCTTTGGAAGTGGTCCC, to reduce the biliary expression of AANAT) (21) or mismatched Morpholino (5'-GTTCCCGACCTTTGCAACTCGTCCC) were designed and purchased from Gene-tools LCC (Philomath, OR, USA) (21). We have previously used this Vivo-Morpholino approach to reduce the biliary expression of AANAT in the liver (21).
Animal models
Male Fischer 344 rats (150-175 gm) were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed at 22 °C with 12:12 hr light/dark cycles. The animals had free access to standard rat chow and drinking water. We used normal and BDL rats that immediately after surgery were treated with Vivo-Morpholino sequences of AANAT or Morpholino mismatched (1 mg/kg BW/day) for one week via an implanted portal vein catheter as described by us (21). To minimize the amount of Vivo-Morpholino that circulates outside of the liver, we used a lower dose (1.0 mg/kg BW/day) (21) of Vivo-Morpholino than that used in a previous study (3.0 mg/kg/day) (23). Animal experiments were performed in accordance with a protocol approved by the Scott & White and Texas A&M Health Science Center IACUC.
Freshly isolated cholangiocytes and immortalized large murine biliary lines
Cholangiocytes (by γ-GT histochemistry) (24) were isolated by immunoaffinity separation (25,26) using an antibody (by Dr. R. Faris, Brown University, Providence, RI, USA) recognizing an unidentified antigen expressed by all intrahepatic cholangiocytes (27). Immortalized large murine cholangiocytes (MCLs, obtained from large bile ducts) (28) display morphological and functional traits similar to that of freshly isolated large cholangiocytes (21,29). MCLs were cultured as described by us (21,29).
Evaluation of VEGF-A/C levels in liver sections, cholangiocytes and total liver lysates
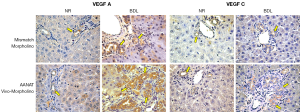
We first evaluated by semi-quantitative immuno-histochemistry (30) the percentage of cholangiocytes positive for VEGF-A/C (key angiogenic factors regulating biliary function) (11,14) in liver sections from the selected groups of rats. Immunohistochemical pictures were taken in a coded fashion by a BX-51 light microscope (Olympus, Tokyo, Japan) with a Videocam (Spot Insight; Diagnostic Instrument, Inc., Sterling Heights, MI, USA) and analyzed with an Image Analysis System (IAS; Delta Sistemi, Rome, Italy). Negative controls were included. The percent of bile ducts positive for VEGF-A/C in liver sections was evaluated as described by us (21,26). When 0-5% of bile ducts were positive we assigned a negative score; a +/- score was assigned when 6-10% of ducts were positive; a + score was assigned when 11-30% of bile ducts were positive (26).
We evaluated by real-time PCR (31) the expression of VEGF-A/C in total RNA of liver tissue and cholangiocytes from rats treated with mismatch or AANAT Vivo-Morpholino for one week. A ΔΔCT analysis was obtained using normal cholangiocytes or normal total liver as control samples (31). The primers for rat VEGF-A/C (SABiosciences) were designed according to the NCBI GenBank Accession numbers: NM_031836 (VEGF-A); and NM_053653 (VEGF-C). Data were expressed as relative mRNA levels ± SEM of the selected gene to glyceraldehyde-3-phosphate dehydrogenase (GAPDH NCBI GenBank Accession NM_017008) ratio.
We next determined VEGF-A/C levels in cholangiocyte and total liver lysates from the selected groups of animals by ELISA kits according to the instructions provided by the vendor.
Overexpression of AANAT in MCL and measurement of VEGF-A/C expression
MCL were transfected using an AANAT cDNA clone vector from OriGene Technologies, Inc. (Rockville, MD, USA) as described by us (21). Transfected cells were selected by the addition of 10 µL/mL geneticin into the media and the selection process was allowed to continue for 4-7 days (21). Surviving cells (MCL-AANAT) were assessed for the relative expression of AANAT compared to the control transfected cells (MCL-puro) by real-time PCR and FACS analysis as described previously by us (21). The clone with the greatest degree of overexpression was selected as described by us (21). In these cells, we measured mRNA of VEGF-A/C by real-time PCR (31) (see above). A ΔΔCT analysis was obtained using MCL-pure as control samples (31). Data were expressed as relative mRNA levels ± SEM of the selected gene to GAPDH ratio.
Statistical analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Effect of AANAT knockdown on the expression of VEGF-A/C levels in liver sections, cholangiocytes and total liver lysates
We have previously validated our model showing that reduction of biliary AANAT expression (by Vivo-Morpholino) was associated with enhanced biliary proliferation and IBDM (21). Concomitant with increased cholangiocyte proliferation, we have shown that the protein expression of VEGF-A/C increased in intrahepatic bile ducts from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls (Figure 1 and Table 1). Furthermore, VEGF-A/C levels were higher in the lysate of total liver and isolated cholangiocytes from BDL rats compared to normal rats (Table 2). VEGF-A/C levels increased in the lysate of total liver and cholangiocytes from both normal and BDL rats treated with AANAT Vivo-Morpholino compared to mismatch-treated rats (Table 2). There was increased expression of the mRNA VEGF-A/C in total liver and cholangiocytes from BDL rats treated with AANAT Vivo-Morpholino compared to control animals (Figure 2A,B).

Full table
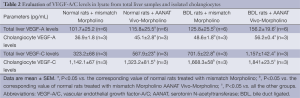
Full table
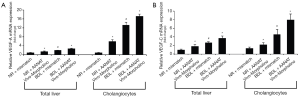
Effect of overexpression of AANAT in MCL on the expression of VEGF-A/C
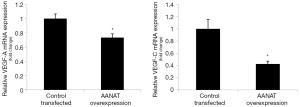
In cholangiocyte lines that stably overexpress AANAT, we demonstrated that there was reduced mRNA expression for VEGF-A/C compared to control cholangiocytes (Figure 3).
Discussion
Our study demonstrates that concomitant with reduced AANAT biliary expression (by in vivo administration of Vivo-Morpholino), there was enhanced immunoreactivity for VEGF-A/C in liver sections from AANAT Vivo-Morpholino treated rats compared to controls. There was increased mRNA expression for VEGF-A/C in total liver samples and cholangiocytes from normal rats treated with AANAT Vivo-Morpholino compared to controls. In vitro overexpression of AANAT in cholangiocytes decreased the biliary expression of VEGF-A/C.
There is growing information regarding the autocrine/paracrine regulation of cholangiocyte proliferation by neuroendocrine factors (e.g., serotonin, VEGF-A/C, secretin and gastrin) which down- and/or up-regulate the growth of the biliary epithelium (10,16,25,32). In a previous study, we found a cross-talk mechanism between cholangiocytes and endothelial cells that mediates the adaptive changes of these cells during liver damage that involves VEGFs (33). The blockage of VEGF secretion decreases cholangiocyte proliferation revealing an autocrine loop, wherein cholangiocytes secrete VEGF interacting with VEGF-R2/R3 to increase biliary proliferation (14). Furthermore, in cholangiocytes from polycystic liver disease samples, VEGF expression is upregulated sustaining cholangiocyte proliferation via autocrine mechanisms (11).
Recently we have also showed the importance of the autocrine role of melatonin (secreted by cholangiocytes) in the regulation of biliary hyperplasia (17,21). In fact, we found that the reduction of biliary AANAT expression and melatonin secretion in cholangiocytes (following AANAT Vivo-Morpholino administration) increases biliary proliferation and increases the expression of SR, CFTR and Cl–/HCO3– AE2, confirming that the AANAT expression and melatonin secretion axis is an important autocrine loop in the local regulation of biliary proliferation (21).
As known, after BDL, the intrahepatic biliary epithelium undergoes cholangiocyte proliferation (4,8,25,34), which leads the expansion of the bile duct mass, which is followed by an adaptive proliferation of the peribiliary plexus (PBP) (15). The PBP origin from the hepatic artery and nourishes the biliary tree (15). Changes in intrahepatic bile duct mass are always associated with changes of the PBP architecture (15,22). After BDL, the increase in intrahepatic bile duct mass is followed by a parallel growth of the PBP (and its circulating factors including VEGF) (15), which is fundamental in sustaining the enhanced nutritional and functional demands of proliferating cholangiocytes (4,8,15,35).
Since proliferation of the PBP follows, in order of time, the proliferation of bile ducts (15), it is reasonable to suppose that proliferating cholangiocytes modulate the adaptive response of the vascular bed. Consistently, proliferating cholangiocytes express VEGF-A/C and secrete VEGFs (14), which modulates cholangiocyte proliferation by autocrine mechanisms. However, we have demonstrated that cholangiocytes VEGFs secretion increases in BDL AANAT-Vivo Morpholino treated rats. The regulation of biliary VEGF-A/C expression by AANAT levels is of particular importance since these two angiogenic factors sustain biliary growth by autocrine mechanisms (14,36). In support of the current findings, the anti-angiogenic activity of melatonin has been demonstrated in advanced cancer patients (37). Also, melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia (38). Since melatonin may affect the angiogenesis of the hepatic microvascularization sustaining biliary functions (33), pharmacological targeting of AANAT may be beneficial for the modulation of biliary disorders.
Acknowledgements
This work was partly supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White Hospital, the NIH grant DK062975 to G.A. and S. G., and a VA Research Career Scientist Award, Research Project funds from University of Rome “La Sapienza”, MIUR grant #2009X84L84_001 and FIRB Accordi di Programma 2010#RBAP10Z7FS to Prof. Gaudio.
Disclosure: The authors declare no conflicts of interest.
References
- Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 1997;272:G1064-74. [PubMed]
- Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, et al. eds. The Liver; Biology & Pathobiology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:421-35.
- Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol 1997;272:G289-97. [PubMed]
- Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 1988;81:569-78. [PubMed]
- Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 2001;281:G612-25. [PubMed]
- Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 2004;127:1565-77. [PubMed]
- Lleo A, Invernizzi P. Apotopes and innate immune system: Novel players in the primary biliary cirrhosis scenario. Dig Liver Dis 2013;45:630-36. [PubMed]
- Alpini G, Glaser SS, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol 1998;274:G767-75. [PubMed]
- LeSage GD, Glaser SS, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol 1999;276:G1289-301. [PubMed]
- Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132:415-31. [PubMed]
- Fabris L, Cadamuro M, Fiorotto R, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 2006;43:1001-12. [PubMed]
- Fabris L, Cadamuro M, Libbrecht L, et al. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology 2008;47:719-28. [PubMed]
- Franchitto A, Onori P, Renzi A, et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med 2013;1:27.
- Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006;130:1270-82. [PubMed]
- Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology 1996;111:1118-24. [PubMed]
- Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005;128:121-37. [PubMed]
- Renzi A, Glaser S, Demorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 2011;301:G634-43. [PubMed]
- Gaudio E, Onori P, Franchitto A, et al. Hepatic microcirculation and cholangiocyte physiopathology. Ital J Anat Embryol 2005;110:71-5. [PubMed]
- Rodríguez Ramírez J, Pena Quintana P, Cabrera Galvan JJ, et al. Severe intrahepatic cholestasis in sepsis caused by Pseudomonas fluorescens. Rev Clin Esp 1989;185:106-7. [PubMed]
- Han Y, Demorrow S, Invernizzi P, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 2011;301:G623-33. [PubMed]
- Renzi A, DeMorrow S, Onori P, et al. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology 2013;57:1130-41. [PubMed]
- Gaudio E, Onori P, Pannarale L, et al. Microcirculation of the extrahepatic biliary tree: a scanning electron microscopy study of corrosion casts. J Anat 1993;182:37-44. [PubMed]
- Arora V, Knapp DC, Reddy MT, et al. Bioavailability and efficacy of antisense morpholino oligomers targeted to c-myc and cytochrome P-450 3A2 following oral administration in rats. J Pharm Sci 2002;91:1009-18. [PubMed]
- Rutenburg AM, Kim H, Fischbein JW, et al. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem 1969;17:517-26. [PubMed]
- Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 2000;32:17-25. [PubMed]
- Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 2009;89:456-69. [PubMed]
- Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology 1989;97:1236-47. [PubMed]
- Ueno Y, Alpini G, Yahagi K, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int 2003;23:449-59. [PubMed]
- Mancinelli R, Franchitto A, Glaser S, et al. GABA induces thee differentiation of small into large cholangiocytes by activation of Ca(2+)/CaMK I-dependent adenylyl cyclase 8. Hepatology 2013;58:251-63. [PubMed]
- Devars du Mayne JF, Deybach JC, Phung L, et al. Acute attacks of hepatic porphyria. Treatment with hematin. 5 cases. Presse Med 1986;15:1673-6. [PubMed]
- Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 2008;295:C499-513. [PubMed]
- Glaser S, Lam IP, Franchitto A, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010;52:204-14. [PubMed]
- Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol 2006;291:G307-17. [PubMed]
- Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 1996;110:1636-43. [PubMed]
- Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology 2002;35:1256-68. [PubMed]
- Spirli C, Okolicsanyi S, Fiorotto R, et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 2010;51:1778-88. [PubMed]
- Lissoni P, Rovelli F, Malugani F, et al. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett 2001;22:45-7. [PubMed]
- Park SY, Jang WJ, Yi EY, et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res 2010;48:178-84. [PubMed]


