
High HbA1c is a risk factor for complications after hepatectomy and influences for hepatocellular carcinoma without HBV and HCV infection
Introduction
Type 2 diabetes mellitus (DM) consists of impaired insulin secretion and/or resistance and therefore causes hyperglycemia. Patients who have liver disease often have concurrent insulin resistance. DM is known to cause complications such as cardiovascular disease, neuropathy, retinopathy and chronic renal failure (1). Furthermore, the number of patients with DM is markedly increasing every year (2). Indeed, the International Diabetes Federation reported that there were 387 million patients with DM worldwide in 2014 and that this number may increase to 592 million by 2035 (3). DM is also a known risk factor for malignancies, such as pancreatic cancer, lung cancer, colon cancer, and hepatocellular carcinoma (HCC) (2,4,5).
Hyperinsulinemia contributes to hepatocarcinogenesis via cell division and proliferation (6,7). Niwa et al. reported that hyperinsulinemia is more strongly related to HCC development than to high blood glucose (8).
Treatment for DM, such as metformin and sodium glucose cotransporter 2 (SGLT2), has been reported to inhibit HCC (9,10).
Hemoglobin A1c (HbA1c) is a biomarker of blood glucose levels over the previous 3 months and is an indicator of diabetes control. However, the impact of HbA1c on the prognosis of resected HCC remains controversial.
Thus, in our current study, we investigated the prognoses and clinicopathological characteristics of HCC patients with high HbA1c levels. We also investigated the surgical outcomes and morbidities of these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.01.03/rc).
Methods
Between January 2001 and December 2018, 771 consecutive patients with HCC underwent primary liver resection at the Gastroenterological Surgery I Unit of the Hokkaido University Hospital in Sapporo, Japan. As we excluded 15 cases with both hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, we analyzed 756 cases.
For stratification, we assigned patients to an HbA1c ≥7.0% group (H-A1c; n=100, 100/756, 13.2%) or an HbA1c <7.0% group (L-A1c; n=656, 656/756, 86.8%) depending on their HbA1c at admission. The Japan Diabetes Society recommends maintaining HbA1c <7.0% to prevent complications (11); thus, we chose an HbA1c of 7 as the cutoff.
We compared prognoses for survival, recurrence, clinicopathological characteristics, surgical outcomes and morbidities between the H-A1c and L-A1c groups. In addition, we evaluated the impact of treatment for DM on the prognoses of HCC patients without HBV and HCV (NBNC patients). HBV included patients with HBV infection alone, HCV included patients with HCV infection alone, and NBNC included patients without HBV and HCV infection.
The indications for hepatic resection and the type of operative procedures were usually determined based on the patients’ liver function reserve, i.e., the results of the indocyanine green retention test at 15 min (ICGR15) (12). Anatomical resection, defined as a resection in which the lesions were completely removed anatomically on the basis of Couinaud’s classification (segmentectomy, sectionectomy, and hemihepatectomy or more), was performed on patients in whom ICGR15 was lower than 25%. Non-anatomical partial but complete resection was achieved in other cases. For all patients, surgery was performed in which the resection surfaces were found to be histologically and macroscopically free of HCC. Follow-up analyses after liver resection were conducted at 3-month intervals, including physical, serological [liver function test, serum alpha-fetoprotein (AFP) level, and serum protein induced by vitamin K absence-II (PIVKA-II)], and radiological [ultrasound sonography (US) and contrast-enhanced computed tomography (CT) scan or contrast-enhanced magnetic resonance imaging (MRI)] examinations. Recurrence was diagnosed on the basis of the results of contrast-enhanced CT and elevation of serum levels of AFP and/or PIVKA-II. Extrahepatic metastasis (lung, lymph node, adrenal gland, brain, and bone) was diagnosed by contrast-enhanced chest and abdominal CT, contrast-enhanced head MRI, and bone scintigram. The median follow-up period was 32 months (range, 1.0-172 months).
Postoperative morbidity was assessed using the validated classification system by Clavien-Dindo (13). Serious complications were categorized as grades III–V and defined as morbidity requiring surgical or radiological intervention. The study was approved by institutional review board of No. 018-0106 and was performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained in the opt-out form on the website of Hokkaido University Hospital.
Statistical analysis
The clinicopathological characteristics of the H-A1c group were compared with those of the L-A1c group. Univariate analyses between two groups were performed using the Mann-Whitney U test for continuous variables and the chi-square test for noncontinuous variables. Multivariate analyses were performed using logistic regression model analysis. Overall survival (OS) and relapse-free survival (RFS) were determined via the Kaplan-Meier method and analyzed with the log-rank test or Cox proportional hazard model. Statistical analyses were performed using JMP Pro 14.0.0 for Windows (SAS Institute, Cary, NC, USA). Significance was defined as a P value of <0.05.
Results
Clinicopathological characteristics of the patients
The NBNC patients with HCC had larger tumor sizes and relatively better liver function than the other patients; in addition, a larger proportion of these patients were undergoing treatment for DM with metformin or other oral drugs (Table 1). In this study, patients using insulin only or insulin combined with other oral drugs except for metformin were defined as the insulin group. Patients using metformin only or metformin combined with other oral drugs were defined as the metformin group. Patients using other oral drugs with neither insulin nor metformin were defined as the other group.
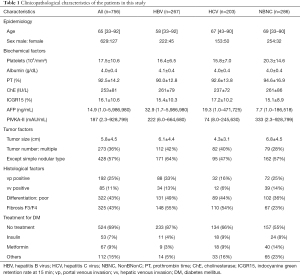
Full table
HbA1c and prognoses in HCC patients in relation to HBV, HCV, and NBNC status
The 5-year OS rates and median survival times (MSTs) of all cases were 65% and 94 months, respectively. The median RFS time was 20 months. Specifically, the underlying liver condition was as follows: 267 cases of HBV, 203 cases of HCV, and 286 cases of NBNC.
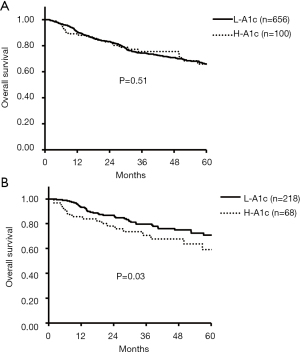
Among all cases, the 5-year OS rates of the H-A1c and L-A1c groups were 65% vs. 65% (P=0.51). For the HBV, HCV, and NBNC groups, the 5-year OS rates of the H-A1c and L-A1c groups were 70% vs. 63% (P=0.78), 48% vs. 66% (P=0.37), and 55% vs. 71% (P=0.03), respectively (Figure 1).
Regarding recurrence, the median RFS time of the H-A1c and L-A1c groups was 16 vs. 21 months (P=0.28) in all cases. In patients with HBV, HCV, and NBNC, the median RFS times of the H-A1c and L-A1c groups were 13 vs. 16 months (P=0.34), 28 vs. 25 months (P=0.62), and 13 vs. 26 months (P=0.02), respectively (Figure 2).
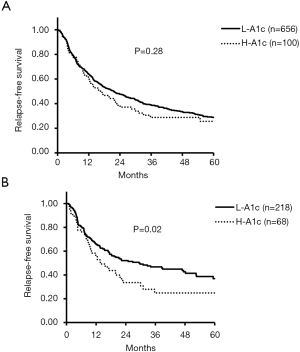
Although both OS and RFS were not significantly different for all cases, HBV cases, and HCV cases, the rates for those in the H-A1c group were significantly more unfavorable in NBNC patients with HCC.
Clinicopathological features of the H-A1c group among NBNC patients with HCC
Table 2 presents the clinicopathological characteristics of the H-A1c and the L-A1c groups of NBNC patients with HCC determined by univariate analyses. No factor except treatment for DM differed between the two groups.
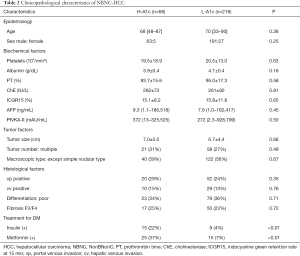
Full table
Univariate and multivariate analyses of OS and RFS among NBNC patients with HCC
Table 3 shows the factors found to influence the OS and RFS in the NBNC cases. Univariate analysis revealed that OS was significantly related to the following: HbA1c ≥7.0%; prothrombin time (PT) <80%; cholinesterase (ChE) <250 IU/L; albumin (Alb) <3.5 g/dL; AFP ≥20 ng/mL; PIVKA-II ≥100 mAU/mL; tumor size ≥5 cm; multiple tumors, except the simple nodular type; poor differentiation; portal venous invasion (vp); hepatic venous invasion (vv); and lack of metformin use. These analyses also indicated that RFS was significantly related to HbA1c ≥7.0%, PT <80%, ChE <250 IU/L, Alb <3.5 g/dL, AFP ≥20 ng/mL, PIVKA-II ≥100 mAU/mL, tumor size ≥5 cm, multiple tumors, except the simple nodular type, poor differentiation, vp, vv, insulin use, and lack of metformin use. Multivariate analysis indicated that ChE <250 IU/L, tumor size ≥5 cm, multiple tumors, vv, and lack of metformin use were independent unfavorable prognostic factors for OS and that PT <80%, ChE <250 IU/L, tumor size ≥5 cm, multiple tumors, vp, vv, insulin use, and lack of metformin use were independent unfavorable prognostic factors for RFS among the patients with NBNC-HCC. HbA1c ≥7.0% was not an independent significant factor for OS or RFS based on multivariate analysis.
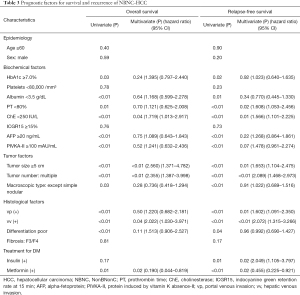
Full table
Prognoses based on treatment for DM in NBNC patients with HCC
Figure 3 shows OS and RFS depending on the treatment for DM.

According to univariate analysis, the patients treated with insulin had significantly poorer RFS rates than the patients treated without insulin (P=0.01) (Table 3); OS was not significantly different. Additionally, the patients treated with metformin showed significantly favorable OS and RFS rates compared with those treated without metformin (P=0.01 and <0.01, respectively). Metformin use was an independent favorable factor for both OS and RFS, whereas insulin use was an independent unfavorable factor for RFS (Table 3). Significant differences were not observed for OS and RFS in patients treated with sulfonylurea (SU), α-glucosidase inhibitor (α-GI), thiazolidine (TZD), and dipeptidyl peptidase 4 inhibitor (DPP-4i).
Surgical outcome and morbidities in all cases
The 30-day and 90-day mortalities of the H-A1c group were 0%; those of the L-A1c group were 0% and 0.1%, respectively. The complications of the patients in the H-A1c group included pleural effusion (5; 5.0%), ascites (7; 7.0%), postoperative bleeding (4; 4.0%), bile leakage (7; 7.0%), hyperbilirubinemia (7; 7.0%), wound infection (4; 4.0%), pneumonia (5; 5.0%), and ileus (2; 2.0%). The complications of the patients in the L-A1c group included pleural effusion (32; 4.9%), ascites (24; 3.7%), postoperative bleeding (22; 3.4%), bile leakage (42; 6.4%), hyperbilirubinemia (18; 2.7%), wound infection (8; 1.2%), pneumonia (10; 1.5%), and ileus (8; 1.2%). The H-A1c group showed significantly higher rates of hyperbilirubinemia (P=0.02), wound infection (P=0.03), and pneumonia (P=0.02). Other complications did not differ significantly. The median postoperative stay times were 15 [3−297] and 15 [5−380] days (P=0.80) for the H-A1c and L-A1c groups, respectively.
The median blood loss was 355 [0−20,190] mL and the median operative time 312 [121−612] minutes in patients with H-A1c; these values were 375 [0−35,820] mL and 312 [78−911] minutes, respectively, for the L-A1c patients. There were no significant differences between the H-A1c and L-A1c groups (Table 4).
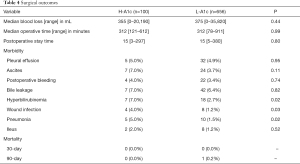
Full table
Discussion
The results of our univariate analysis indicate that high HbA1c is a significantly unfavorable factor for survival and recurrence in NBNC patients with HCC. However, there were no differences between patients with HBV or HCV. In this cohort of NBNC patients, those treated with insulin showed significantly poorer RFS than those treated without insulin. Conversely, the patients treated with metformin showed significantly favorable OS and RFS rates compared with the patients treated without metformin. Regarding postoperative morbidities, there were significantly higher rates of hyperbilirubinemia, wound infection, and pneumonia in the H-A1c group than in the L-A1c group.
Type 2 DM is known as a risk factor for several malignancies (2,4,5). In particular, there have been many reports showing a correlation between type 2 DM and HCC. Li et al. reported that DM was associated with an increased risk for HCC in HCV patients (14), and DM was found to be a risk factor for HCC in patients with HCV who had mild (not severe) fibrosis and a sustained virologic response (15). Huang et al. also reported that metabolic risk factors such as fatty liver, high triglyceride levels and a history of DM are significantly associated with NBNC-HCC (16).
In a meta-analysis, Wang et al. found that DM was independently associated with poorer survival and recurrence in HCC (17), whereas Howell et al. reported that DM did not negatively impact the prognosis of HCC (18). Whether DM is a prognostic factor for resected HCC remains controversial. In this study, high HbA1c was a significantly unfavorable factor for survival and recurrence in NBNC-HCC, as based on univariate analysis. However, it was not an independent factor according to multivariate analysis. Shau et al. reported that DM is an independent factor for poorer prognosis in patients who received curative therapy for HCC, such as surgery or ablation, but these authors did not mention recurrence (19). Additionally, Wang et al. showed that DM did not significantly affect the disease-free survival of patients with HCC after curative hepatectomy but that DM was associated with significantly lower OS (20). Our study showed no significant difference in OS and RFS among all cases. This discrepancy may be due to the status of the hepatitis virus (HBV and HCV) infection. These studies, including ours, included different proportions of HBV, HCV, and NBNC cohorts. Kawamura et al. reported that DM is a significant predictor of tumor recurrence after curative therapy for HCC in patients with nonviral hepatitis (21), consistent with the findings in our study. He et al. also indicated that the HbA1c-based score model can be used to predict death risk in patients with HCC and type 2 DM (22). Among patients with nonalcoholic fatty liver disease (NAFLD), insulin resistance influences inflammation, from which HCC may develop (23). Thus, high HbA1c might more strongly influence the prognosis of patients with NBNC-HCC than those with viral hepatitis.
Exogenous insulin is thought to promote oncogenesis by increasing insulin-like growth factor 1 (IGF-1) activity. A meta-analysis by Singh et al. showed that the use of insulin was associated with a statistically significant (161%) increase in the risk of HCC (24). Regarding the mechanism of DM development in HCC, several studies have advocated that hyperinsulinemia contributes to hepatocarcinogenesis via cell division and proliferation (6,7). Insulin promotes the phosphorylation of insulin receptor substrate-1 (IRS-1) and transmission of the insulin signal through the phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) cascades (6), possibly leading to abnormal cell proliferation and metabolism (25). Nonetheless, it remains unclear how those factors can affect HCC after curative therapy. Zheng et al. revealed that the activation of AMPK by metformin inhibits NF-kB and STAT3 signaling and thus suppresses HCC cell growth (26). Additionally, metformin is able to inhibit HCC cell growth by regulating cell-cycle regulatory proteins, such as cyclin D1 and cyclin E. Metformin may also inhibit c-myc expression by upregulating the let-7 family (27). Some researchers have reported that metformin might reduce the risk of recurrence or cancer-related mortality after curative resection of HCC (28,29). Tseng et al. also reported that metformin was associated with a reduced risk of HCC, depending on the cumulative duration of use (30). In the present study, the use of insulin had an unfavorable impact on recurrence, though the use of metformin had a favorable impact on both recurrence and survival. Thus, we might consider the use of metformin for patients with NBNC-HCC after hepatectomy.
Lee et al. reported that DM was a risk factor for major complications after hepatectomy (31), yet their study did not show the details of these complications. A meta-analysis by Li et al. revealed no significant difference in total complications after hepatectomy between patients with DM and those without DM, but liver failure, ascites, and postoperative infection were significantly higher in patients with DM (32). In line with these findings, in the present study, there was no significant difference in total complications after hepatectomy, whereas the rates of hyperbilirubinemia, wound infection, and pneumonia were significantly higher in the H-A1c group than in the L-A1c group. Patients with DM are susceptible to infection (33), and the higher rates of wound infection and pneumonia appear to be reasonable findings. DM has been suggested to exacerbate existing liver fibrosis, resulting in severe liver failure via increased expression of connective tissue growth factor or the production of reactive oxygen species (20). Thus, hyperbilirubinemia might be highly prevalent in the H-A1c group.
However, there are some limitations of this study, which was a completely retrospective study. The number of H-A1c patients was also low compared with the total number of patients.
Conclusions
High HbA1c alone may not be an independent prognostic factor for HCC. Maintaining goal blood glucose levels over the 3 months prior to surgery might be favorable for the prognosis of NBNC patients with HCC, and metformin might be a beneficial drug for patients with HCC after hepatectomy. In addition, high HbA1c may be a risk factor for morbidities after hepatectomy.
Acknowledgments
The authors would like to thank the staff of the Gastroenterological Surgery I Unit of the Hokkaido University Graduate School of Medicine for their cooperation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.01.03/rc
Data Sharing Statement: Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.01.03/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.01.03/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional review board of No. 018-0106 and was performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained in the opt-out form on the website of Hokkaido University Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rahimi Z, Moradi M, Nasri H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J Res Med Sci 2014;19:1090-8. [PubMed]
- Noto H, Osame K, Sasazuki T, et al. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications 2010;24:345-53. [Crossref] [PubMed]
- International Diabetes Federation. IDF Diabetes atlas. Diabetes atlas. 2015. Available online: http://www.idf.org/
- Fang HJ, Shan SB, Zhou YH, et al. Diabetes mellitus and the risk of gastrointestinal cancer in women compared with men: a meta-analysis of cohort studies. BMC Cancer 2018;18:422. [Crossref] [PubMed]
- Tseng CH. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget 2017;8:41132-42. [Crossref] [PubMed]
- Sasaki Y. Insulin resistance and hepatocarcinogenesis. Clin J Gastroenterol 2010;3:271-8. [Crossref] [PubMed]
- Chettouh H, Lequoy M, Fartoux L, et al. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int 2015;35:2203-17. [Crossref] [PubMed]
- Niwa Y, Ishikawa K, Ishigami M, et al. Effect of hyperglycemia on hepatocellular carcinoma development in diabetes. Biochem Biophys Res Commun 2015;463:344-50. [Crossref] [PubMed]
- Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881-91. [Crossref] [PubMed]
- Kaji K, Nishimura N, Seki K, et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer 2018;142:1712-22. [Crossref] [PubMed]
- Japan Diabetes Society. editors. Treatment Guide for Diabetes 2016-2017. Bunkodo Tokyo.
- Kamiyama T, Nakanishi K, Yokoo H, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg 2010;211:443-9. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Li X, Xu H, Gao Y, et al. Diabetes mellitus increases the risk of hepatocellular carcinoma in treatment-naive chronic hepatitis C patients in China. Medicine (Baltimore) 2017;96:e6508 [Crossref] [PubMed]
- Huang CF, Yeh ML, Huang CY, et al. Pretreatment glucose status determines HCC development in HCV patients with mild liver disease after curative antiviral therapy. Medicine (Baltimore) 2016;95:e4157 [Crossref] [PubMed]
- Huang SF, Chang IC, Hong CC, et al. Metabolic risk factors are associated with non-hepatitis B non-hepatitis C hepatocellular carcinoma in Taiwan, an endemic area of chronic hepatitis B. Hepatol Commun 2018;2:747-59. [Crossref] [PubMed]
- Wang YG, Wang P, Wang B, et al. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One 2014;9:e95485 [Crossref] [PubMed]
- Howell J, Yiu M, Gibson R, et al. Type 2 diabetes does not worsen prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2011;35:214-20. [Crossref] [PubMed]
- Shau WY, Shao YY, Yeh YC, et al. Diabetes mellitus is associated with increased mortality in patients receiving curative therapy for hepatocellular carcinoma. Oncologist 2012;17:856-62. [Crossref] [PubMed]
- Wang YY, Huang S, Zhong JH, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One 2014;9:e113858 [Crossref] [PubMed]
- Kawamura Y, Ikeda K, Arase Y, et al. Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol 2008;23:1739-46. [Crossref] [PubMed]
- He L, Zhang S, Liu X, et al. HbA1c-based score model for predicting death risk in patients with hepatocellular carcinoma and type 2 diabetes mellitus. J Diabetes Res 2017;2017:3819502 [Crossref] [PubMed]
- Chen K, Ma J, Jia X, et al. Advancing the understanding NAFLD to hepatocellular carcinoma development: From experimental models to humans. Biochim Biophys Acta Rev Cancer 2019;1871:117-25. [Crossref] [PubMed]
- Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881-91. [Crossref] [PubMed]
- Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254-8. [Crossref] [PubMed]
- Zheng L, Yang W, Wu F, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res 2013;19:5372-80. [Crossref] [PubMed]
- Li J, Hernanda PY, Bramer WM, et al. Anti-tumor effects of metformin in animal models of hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One 2015;10:e0127967 [Crossref] [PubMed]
- Chan KM, Kuo CF, Hsu JT, et al. Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int 2017;37:434-41. [Crossref] [PubMed]
- Seo YS, Kim YJ, Kim MS, et al. Association of metformin use with cancer-specific mortality in hepatocellular carcinoma after curative resection: a nationwide population-based study. Medicine (Baltimore) 2016;95:e3527 [Crossref] [PubMed]
- Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int 2018;38:2018-27. [Crossref] [PubMed]
- Lee CW, Tsai HI, Sung CM, et al. Risk factors for early mortality after hepatectomy for hepatocellular carcinoma. Medicine (Baltimore) 2016;95:e5028 [Crossref] [PubMed]
- Li Q, Wang Y, Ma T, et al. Clinical outcomes of patients with and without diabetes mellitus after hepatectomy: A systematic review and meta-analysis. PLoS One 2017;12:e0171129 [Crossref] [PubMed]
- Tsai MS, Lin CL, Chang SN, et al. Diabetes mellitus and increased postoperative risk of acute renal failure after hepatectomy for hepatocellular carcinoma: a nationwide population-based study. Ann Surg Oncol 2014;21:3810-6. [Crossref] [PubMed]


