Coronavirus disease 2019: implications for liver transplantation
The Coronavirus disease 2019 (COVID-19) has become a worldwide pandemic. The etiology was identified as a new corona virus, recently named severe acute respiratory syndrome coronavirus 2 (SRAS-CoV-2). Whether patients in incubation period and asymptomatic infected patients have the ability to infect others remains unclear, and the severity of symptoms appears to vary in different regions, which brings great challenges to the preventive work of this outbreak. Therefore, the Chinese government has begun policies recently to prevent the spread of asymptomatic infections. As declared by the World Health Organization, the total confirmed cases and the total death cases have reached 1,699,595 and 106,138 worldwide, respectively, as of April 13, 2020, and these numbers are stilling growing rapidly.
Liver is commonly affected by SRAS-CoV-2
SARS-CoV-2 has been detected in blood, stool and urine from infected patients (1,2), which implies that the virus is not restricted to the respiratory system and could affect multiple organs. Liver is one of the organs most commonly affected by SRAS-CoV-2 according to the accumulated knowledge (Table 1) (3-8). Recent studies have reported that some COVID-19 patients developed liver dysfunction, and the degree of liver dysfunction is related to the severity of SRAS-CoV-2 infection (6,7). Another comparative study showed that patients with SRAS-CoV-2 infection have higher levels of liver damage-related biomarkers, such as aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transpeptidase, than patients with other pneumonia (5). All this evidence implies the potential exposure of the liver to the SRAS-CoV-2 virus.

Full table
Challenge for liver transplant (LT) recipients
Considering the easy transmissibility and liver-damage effect of the SARS-CoV-2, LT recipients, who are in a special immune imbalance due to the use of immunosuppressants, might be more vulnerable to the virus. In a recent case report of SARS-CoV-2 infection in a 37-year-old male LT patient (9), to achieve a balance between graft rejection and viral infection, doctors adjusted the dosage of immunosuppressants, but this is followed by repeated abnormal liver function or viral infections. Another case also suggested that reduction or temporary removal of immunosuppressive agents might be beneficial for LT patients with COVID-19 (10). These facts imply an urgent problem that how immunosuppression strategies can be optimized, especially during epidemics.
LT recipients infected by SARS-CoV-2 might have poor prognosis. As we know, hypertension and diabetes mellitus are common metabolic complications after LT, which may affect the prognosis of recipients (11,12). A phenomenon that needs attention is metabolic diseases (hypertension and diabetes) are common in hospitalized COVID-19 patients in China, especially in the dead. This implies that LT recipients infected by SARS-CoV-2 might be more likely to develop metabolic complications and therefore have poorer prognosis. By now, there is still a lack of effective treatments for COVID-19. Lopinavir/ritonavir combined with arbidol—is widely adopted to eliminate the virus. However, abnormal liver function was observed among COVID-19 patients receiving that treatment. Therefore, from the virus itself to the treatment of the virus, the liver may always be damaged.
However, whether LT is a risk factor of SARS-CoV-2 infection is still uncertain, since there are few reports of SARS-CoV-2 infection in LT patients by now. This might due to the immunotherapies which prevent inflammatory responses, making the clinical symptoms of the early stage of infection not obvious and hindering the diagnosis. Although there is insufficient clinical evidence, it is reasonable to believe that LT patients are more likely to be infected in this outbreak. Therefore, we must pay more attention to the management of LT in this special time. After all, prevention is always better than remedy.
Prevention of being infected
Up to now, little is known about donor-to-recipient transmission of SARS-CoV-2 in LT surgery. However, in order to protect the safety of patients, we must try to eliminate possible donor-derived transmissions. Pan and colleagues recently well described their protocols to prevent transmission of this virus during organ donation, including pre-transplant and post-transplant strategies (13). In spite of that, patients in incubation period present new challenges for the management of organ donation.
Whether liver grafts can be directly infected by the virus still remains unclear. SRAS-CoV-2 enters human cells through binding angiotensin converting enzyme (ACE) 2, which is a receptor mainly expressed in bile duct epithelial cells in liver tissues but less expressed in hepatocytes. However, in the process of hepatic repair after injury, bile duct epithelial cells expressing ACE2 might dedifferentiate and proliferate into liver cells, which might partly account for the liver injury in COVID-19 patients (14). Researchers still have no idea about whether liver grafts from infected donors can be used safely. Apparently the safest approach is to suspend or reduce living donation in severely affected areas. LTs with a clear risk of infection should be strictly avoided. For those severe patients who urgently need LT, rigorous test on the donors are suggested. In addition, testing may also be necessary during the ex-vivo preservation of grafts. In short, the principle of LT operation during the epidemic is to create a relatively isolated virus-free environment and ensure that each participant does not carry the virus.
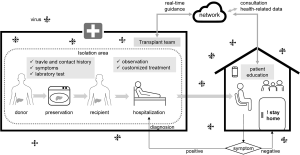
Preventing post-LT recipients from being infected from outside the hospital is necessary. We are aware that there is still a lack of an electronic information system that guides transplant recipients in real time on how to respond to infectious diseases (Figure 1). For those recipients who can still be contacted, we could develop a specific guideline for their prevention and response to the epidemic, and send this to them through network. The guidelines should be developed for different types of patients, guiding them to decide whether to adjust their immunosuppressive regimen based on their physical conditions and living environment. Although there is little evidence for how to build such a complete set of guidelines, we can improve as we go, and try to provide the most timely guidelines for patients.
How to deal with infected recipients
Special treatments focusing on protecting livers might be required for these patients. The combination of multiple antiviral drugs might leads to abnormal liver function. Since mainstream antiviral drugs such as lopinavir and ritonavir need to be metabolized by enzymes in the liver, the inappropriate combination of these drugs may cause interactions and thus harm the liver. Therefore, for the treatment of infected LT recipients, unnecessary combination of drugs should be avoided. And remdesivir, a broad-spectrum antiviral nucleotide prodrug, might be a promising treatment (15,16).
Blocking cytokine storms tend to become a promising treatment for rescuing severe COVID-19 patients. Based on this fact, tocilizumab, a blocker of IL-6R, might become an effective drug for severe COVID-19 patients (17). However, considering the immunosuppressive status of LT recipients, the cytokine storms might not happen in them. So for a LT recipients with severe COVID-19, primary immunotherapies might be beneficial, and tocilizumab seems to be less effective in these patients.
Inhibiting the process of ACE2-involved cell entry might be a promising strategy for blocking SARS-CoV-2 infection. An interesting fact is that metabolic complications (hypertension and diabetes mellitus) are common after LT, that often requires ACE inhibitors and angiotensin II type I receptor blockers (reducing blood pressure), which may lead to overexpression of ACE2 (18). This suggests that LT recipients with metabolic complications might be more likely to develop COVID-19, and more evidence is needed in this regard. In addition, for infected recipients with indications of LT, retransplantation may not be a good strategy, because the virus still exists in the body of recipient, and the stress caused by retransplantation may be beyond the recipient’s tolerance. In extremely severe cases, consideration may be given to participating in clinical trials.
Summary and outlook
Although it can be inferred by common sense that LT recipients might be more vulnerable to SARS-CoV-2 virus, as yet, there is no reports about whether LT is a risk factor for COVID-19. Considering the liver-damage effects of the COVID-19 and the treatments, and the potential protective effect on infected recipients of immunosuppressants by suppressing cytokine storm, the actual circumstances might be complicated for doctors. We encourage the establishment of an electronic information system to keep in touch with all LT recipients to guide them in emergency situations. This is challenging work. This epidemic has shown us what the era of globalization really is, and we transplant workers should also be more up-to-date and work together with counterparts from all countries to improve transplant strategies in an emergency.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Funds for Distinguished Young Scholar of China (81625003), the National Natural Science Foundation of China (81570589, 81800578, 81930016), the National Science and Technology Major Project (2017ZX10203205).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn-20-447/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020;323:1488-94. [Crossref] [PubMed]
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514-23. [Crossref] [PubMed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Wang L, He W, Yu X, et al. Coronavirus Disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis 2020. [Epub ahead of print]. [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Cui Y, Tian M, Huang D, et al. A 55-Day-Old Female Infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis 2020;221:1775-81. [Crossref] [PubMed]
- Qin J, Wang H, Qin X, et al. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Liu B, Wang Y, Zhao Y, et al. Successful Treatment of Severe COVID-19 Pneumonia in a Liver Transplant Recipient. Am J Transplant 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Ling Q, Xie H, Lu D, et al. Association between donor and recipient TCF7L2 gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation in a Han Chinese population. J Hepatol 2013;58:271-7. [Crossref] [PubMed]
- Ling Q, Xie H, Li J, et al. Donor Graft MicroRNAs: A Newly Identified Player in the Development of New-onset Diabetes After Liver Transplantation. Am J Transplant 2017;17:255-64. [Crossref] [PubMed]
- Pan L, Zeng J, Yang H. Challenges and countermeasures for organ donation during the SARS-CoV-2 epidemic: the experience of Sichuan Provincial People’s Hospital. Intensive Care Med 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Xie H, Zhao J, Lian N, et al. Clinical Characteristics of non-ICU Hospitalized Patients With Coronavirus Disease 2019 and Liver Injury: A Retrospective Study. Liver Int 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020;382:929-36. [Crossref] [PubMed]
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269-71. [Crossref] [PubMed]
- Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Boeckmans J, Rodrigues RM, Demuyser T, et al. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol 2020. [Epub ahead of print]. [Crossref] [PubMed]