Ruptured choledochal cyst: a rare presentation and unique approach to management
Introduction
Biliary cysts (BC) are congenital cystic dilatations of the intra- and/or extra-hepatic biliary tree that are exceedingly rare in North America. Incidence is geographically dependent, being more common in Asia (1/1,000) than in western countries (1/100,000-150,000). Women are more commonly affected than men in a 3-4:1 ratio (1). Traditionally, lesions affecting only the common bile duct (CBD) are referred to specifically as choledochal cysts (CC). In 1969, Babbitt established that the most probable cause of BC relies on an anomalous pancreatobiliary junction (APBJ) (2). According to Yotuyangi, an anomalous proliferation of the biliary epithelial cells before bile duct cannulation during development is one possible explanation (3). The most well-known classification system for BCs was established by Todani (4). There are five types, type I being the most common. Types I, II, III, and IVb are extra-hepatic and type IVa and V are intra-hepatic. BCs have the ability to produce a wide spectrum of complications, including strictures, cholangitis, rupture with biliary peritonitis, and malignancy (5). In this paper, we present the rare case of a ruptured CC managed conservatively at first, followed by elective resection after resolution of biliary peritonitis.
Case report
An 18-year-old female presented to the Emergency Department with a 2-day history of abdominal pain, nausea and vomiting. Vital signs were stable with the exception of mild fever (38.5 °C). On examination, the patient looked slightly jaundiced and the abdomen was tender on palpation in the right upper quadrant. Past medical history revealed a similar episode 1 year earlier that settled without complication and was never investigated further.
Initial blood work revealed a picture of obstructive jaundice, with an elevated WBC (25.8×109 cells/L), total and conjugated bilirubin (72 & 49 µmol/L respectively), and liver enzymes (AST 60 IU/L, ALT 133 IU/L, ALP 146 IU/L, GGT 165 IU/L). Lipase was also elevated (200 IU/L), while serum amylase, lactate, creatinine, and electrolytes fell within normal limits. Beta-hCG was negative.
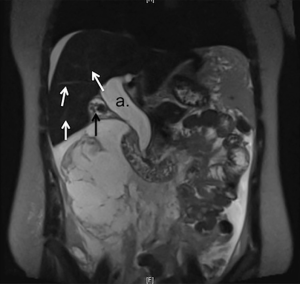
Ultrasound revealed free fluid in the abdominal cavity, a contracted gallbladder and a dilated CBD. The patient was sent for CT, which showed a large fluid collection extending from the pericholecystic region to the pelvis and collecting in the right paracolic gutter (Figure 1). Furthermore, fluid could be identified tracking retroperitoneally. The CBD dilatation was measured at 3.2 cm in diameter. A MRCP study further characterized the severe CBD dilation with distal tapering and a normal intra-hepatic biliary tree, confirming the diagnosis of CC type I (Figure 2).

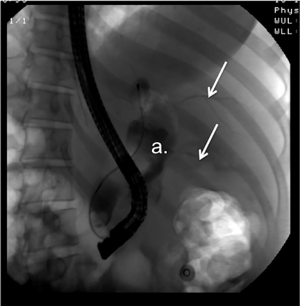
The patient was admitted and referred to HPB Surgery. A diagnostic paracentesis revealed clear bile. Intravenous fluids and broad-spectrum antibiotics were given, a nasogastric tube was placed and two percutaneous intra-abdominal drains were placed by Interventional Radiology, one sub-hepatic and one inferiorly into the pelvis. An ERCP was performed, including the removal of two small cholesterol stones and a sphincterotomy to relieve pressure in the cyst and promote sealing of the rupture. There was no radiological evidence of bilious leakage from the CBD (Figure 3). The patient did well after the procedure and was discharged home after a few days of observation. The plan was to continue conservative management for 6-8 weeks, avoiding laparotomy and definitive surgical management until the perforation healed and the bili-peritoneum had resolved. The percutaneous drains were left in and continued to put out minimal biliary fluid over the next 6 weeks.
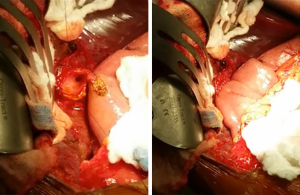
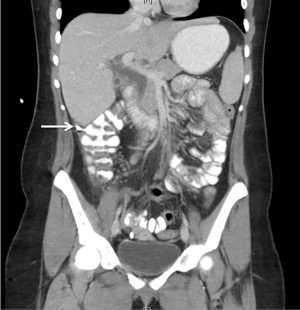
A follow-up CT performed 6 weeks later showed resolution of the bili-peritoneum and decompression of the CC (Figure 4), establishing the success of initial conservative management. The patient was scheduled for elective resection and reconstruction at 8 weeks after initial presentation. The abdomen was accessed through a right subcostal incision and the anatomy was revealed through minimal adhesions. Exploratory laparotomy revealed small collections of bile around the percutaneous drains. The cyst was identified and no other areas of abnormality were visualized. The likely site of rupture was identified within the CBD as an area of fibrosis and thinning of the wall. Mobilization of the duodenum was performed through a Kocher maneuver. The portal vascular structures were identified to avoid injury. Excision of the cyst as close to the pancreatic junction as possible, together with cholecystectomy, was performed with minimal blood loss. Intrahepatic hepaticojejunostomy was performed through a Roux-en-Y jejunal limb (Figure 5). The patient did well after surgery with no evidence of leak and was discharged home on post-operative day 5.
Pathology reported an acute on chronic cholecystitis with cholelithiasis. Inflammatory atypia of surface epithelium of the CC was noted with no evidence of malignancy. The patient was seen in clinic 4 weeks after surgery with no complications. She remains asymptomatic with normal liver function 1 year later and continues to be followed annually.
Discussion
Biliary peritonitis secondary to rupture of a CC is a rare presentation described in less than two percent of cases (6-8). Patients usually present as children (only about 20-30% present as adults) with the classic triad of palpable abdominal masses, pain in right upper quadrant and jaundice (9,10). BCs may present with different types of complications, including cystolithiasis, cholangitis, pancreatitis, and malignancy; the later has a 20-fold increased risk in BCs compared with the general population and increases with age (11).
The underlying cause for BCs is unknown. The most accepted theory involves an APBJ, which is found in 50-80% of BCs (2). In APBJ, the distal portion of the CBD and the pancreatic duct are formed 1-2 cm proximal to the ampulla of Vater and sphincter of Oddi, creating a large common channel where biliary and pancreatic secretions can pool. Due to greater secreting pressures in the pancreas, this mixture of secretions can reflux into the biliary tree. The biliary juices activate the pancreatic enzymes, creating an inflammatory environment which weakens the mucosa and dilates the walls of the CBD, thus making it more prone to cystic degeneration, dysplasia and eventually malignancy (12). In this case, however, imaging revealed a normal APBJ.
BC classification started in 1959 with Alonso et al., in which they described only three extra-hepatic types of cysts. Later in 1977, Todani et al. included intrahepatic cysts and by 2003 the classification was refined once more to include APBJs. Presently, the most commonly used classification is Todani et al. modified version which consists of five types and subtypes of cysts (Table 1) (13,15).
Risk factors predisposing to rupture are not well described in the literature. In this case, ERCP revealed multiple stones in the distal CBD as well as fibrosis and inflammatory atypia. It is possible that increased pressure and inflammation of the cyst as a result of choledocholithiasis predisposed this patient to spontaneous rupture.
Imaging is necessary to make the correct diagnosis of ruptured CC. This is especially true in the setting of acute rupture and biliary peritonitis where an accurate diagnosis may call for conservative management and avoidance of emergency surgery. Ultrasound is often the first imaging tool used because of its lack of invasiveness, availability and low cost, but CT is more sensitive at visualizing the intrahepatic ducts (14,16).
Both ERCP and MRCP are highly sensitive at confirming the diagnosis (17), although MRCP is preferred due to the invasiveness and risk of complications (pancreatitis, hemorrhage, perforation) associated with ERCP (18). However, ERCP potentiates the ability to conservatively manage a ruptured cyst through sphincterotomy or papillotomy, as was done in this case.
Over the years there has been a wide variety of approaches to management of ruptured CC. From 1960 to the 1980s, the treatment of choice was laparotomy and external biliary drainage with excision and biliary reconstruction occurring months to years later (19,20). However, in the 1990s many believed that the best management involved performing a single surgery at the moment of the BC rupture (21).
In recent years, the literature on the topic has changed again. Franga et al. in 2013 and Fragulidis et al. in 2007, stated that the best-suited approach to a BC rupture was to perform a laparotomy-guided T-tube drainage followed by an interval cystectomy and hepaticoenterostomy (18,22). In 2008, Kang et al. suggested that a percutaneous trans-hepatic cyst drainage preceding definite surgery was the best option for BC rupture with peritonitis (23). In most cases, cystenterostomy (internal drainage) of the BC alone has been abandoned due to its high risk of malignancy, which has been reported in as many as 50% of cases (24).
A single stage surgery may be a suitable treatment for patients who are stable and free of other immediate complications. However, in a patient presenting with acute rupture and peritonitis, the risk of complications during definitive surgical management may be too high. In the acute setting, the placement of a T-tube and delayed excision with biliary reconstruction may be appropriate for patients that are not in optimal condition to tolerate surgery. However, due to the fact that this option consists of two surgeries, additional comorbidities may exist.
Conclusions
This case highlights the benefit of an accurate diagnosis and initial conservative management of BC rupture. Drainage of the peritoneal fluid and ERCP-guided sphincterotomy allowed decompression and temporary seal of the rupture. The benefit of a single elective surgery after resolution of peritonitis may be significant to reduce complications and improve long-term outcomes involving biliary function.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Informed Consent: Informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Singham J, Yoshida EM, Scudamore CH. Choledochal cysts: part 1 of 3: classification and pathogenesis. Can J Surg 2009;52:434-40. [PubMed]
- Santiago I, Loureiro R, Curvo-Semedo L, et al. Congenital cystic lesions of the biliary tree. AJR Am J Roentgenol 2012;198:825-35. [PubMed]
- Yotuyangi S. Contribution to etiology and pathology of idiopathic cystic dilatation of the common bile duct, with a report of three cases. Gann (Tokyo) 1936;30:601.
- Dumitrascu T, Lupescu I, Ionescu M. The Todani classification for bile duct cysts: an overview. Acta Chir Belg 2012;112:340-5. [PubMed]
- Wiseman K, Buczkowski AK, Chung SW, et al. Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg 2005;189:527-31; discussion 531. [PubMed]
- Saluja SS, Nayeem M, Sharma BC, et al. Management of choledochal cysts and their complications. Am Surg 2012;78:284-90. [PubMed]
- Yamaguchi M. Congenital choledochal cyst. Analysis of 1,433 patients in the Japanese literature. Am J Surg 1980;140:653-7. [PubMed]
- Treem WR, Hyams JS, McGowan GS, et al. Spontaneous rupture of a choledochal cyst: clues to diagnosis and etiology. J Pediatr Gastroenterol Nutr 1991;13:301-6. [PubMed]
- Abbas HM, Yassin NA, Ammori BJ. Laparoscopic resection of type I choledochal cyst in an adult and Roux-en-Y hepaticojejunostomy: a case report and literature review. Surg Laparosc Endosc Percutan Tech 2006;16:439-44. [PubMed]
- Alonso-Lej F, Rever WB Jr, Pessagno DJ. Congenital choledochal cyst, with a report of 2, and an analysis of 94, cases. Int Abstr Surg 1959;108:1-30. [PubMed]
- Søreide K, Søreide JA. Bile duct cyst as precursor to biliary tract cancer. Ann Surg Oncol 2007;14:1200-11. [PubMed]
- Babbitt DP. Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb. Ann Radiol (Paris) 1969;12:231-40. [PubMed]
- Jabłońska B. Biliary cysts: etiology, diagnosis and management. World J Gastroenterol 2012;18:4801-10. [PubMed]
- Singham J, Yoshida EM, Scudamore CH. Choledochal cysts: part 2 of 3: Diagnosis. Can J Surg 2009;52:506-11. [PubMed]
- Todani T, Watanabe Y, Toki A, et al. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg 2003;10:340-4. [PubMed]
- Lam WW, Lam TP, Saing H, et al. MR cholangiography and CT cholangiography of pediatric patients with choledochal cysts. AJR Am J Roentgenol 1999;173:401-5. [PubMed]
- Bhavsar MS, Vora HB, Giriyappa VH. Choledochal cysts: a review of literature. Saudi J Gastroenterol 2012;18:230-6. [PubMed]
- Franga DL, Howell CG, Mellinger JD, et al. Single-stage reconstruction of perforated choledochal cyst: case report and review of the literature. Am Surg 2005;71:398-401. [PubMed]
- Ando H, Ito T, Watanabe Y, et al. Spontaneous perforation of choledochal cyst. J Am Coll Surg 1995;181:125-8. [PubMed]
- Friend WD. Rupture of choledochal cyst during confinement. Br J Surg 1958;46:155-7. [PubMed]
- Moss RL, Musemeche CA. Successful management of ruptured choledochal cyst by primary cyst excision and biliary reconstruction. J Pediatr Surg 1997;32:1490-1. [PubMed]
- Fragulidis GP, Marinis AD, Anastasopoulos GV, et al. Management of a ruptured bile duct cyst. J Hepatobiliary Pancreat Surg 2007;14:194-6. [PubMed]
- Kang CM, Lee KH, Kim DH, et al. Percutaneous transhepatic cyst drainage as a “bridge procedure” to definitive treatment of perforated choledochal cysts: a case report. Surg Laparosc Endosc Percutan Tech 2008;18:598-600. [PubMed]
- Watanabe Y, Toki A, Todani T. Bile duct cancer developed after cyst excision for choledochal cyst. J Hepatobiliary Pancreat Surg 1999;6:207-12. [PubMed]