Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a rising worldwide public health problem characterized by a typical sequence of disease stages. Early stages involve lipid accumulation (steatosis) and inflammation, which may proceed to chronic inflammation and compensatory tissue repair, leading to accumulation of collagen and scarring (fibrosis or cirrhosis). Liver cirrhosis is associated with progressive loss of organ function and forms the basis for hepatocellular carcinoma (HCC) development. At a certain stage, liver cirrhosis and HCC are non-reversible, often leaving organ transplantation as the only therapeutic option (1-3). Understanding the molecular mechanisms that control the transition between the disease stages is thus critical for the design of disease-modifying therapies.
In Western countries, the metabolic syndrome was found to be a strong predictor of NAFLD development. Obesity, diabetes, insulin resistance and hyperlipidemia, which are the core characteristic features of the metabolic syndrome, are strongly associated with NAFLD (4-6). The prevalence of non-alcoholic steatohepatitis (NASH), which is characterized by the development of liver inflammation, among a general medical population diagnosed with NAFLD is 30%. These patients have a high likelihood of developing advanced fibrosis and cirrhosis (7,8). Although inflammation is considered as main contributor to hepatic tissue damage during NAFLD progression, it is not well understood how the inflammatory mechanisms are initiated. Our current knowledge suggests that the complex local interactions between the cellular components of the innate immune system and the resident cell types of the liver play an essential role in perpetuating and modulating the inflammatory response in the liver.
The innate immune cells in the liver recognize cell damage or pathogen invasion with intracellular or surface-expressed pattern recognition receptors (PRRs). These receptors detect either damage-associated molecular patterns (DAMPs) released from injured cell, or pathogen-associated molecular patterns (PAMPs) which can be derived from gut-derived microbial products (9,10). Therefore, our working hypothesis is that these DAMPs and PAMPs can activate the innate immune system during NAFLD progression, thereby subsequently initiating signaling cascades that trigger the release of factors promoting the inflammatory response. In this review, we will focus on innate immune signaling, gut-liver interactions and the metabolic consequences during NAFLD progression.
PRR signaling during NAFLD
Toll-like receptors (TLRs) and NOD-like receptors (NLRs) are well known examples of PRRs. The activation of these PRRs induces the transcription of genes involved in the innate immune responses (9,10). Therefore, PRR signaling can play an important role during NAFLD development.
Toll-like receptors (TLRs)
TLRs are expressed on Kupffer cells, endothelial cells, dendritic cells, biliary epithelial cells, stellate cells and hepatocytes in liver. They recognize a wide variety of PAMPs, thereby activating these different cell types and contributing to the inflammatory response. TLRs are structurally characterized by the presence of an individual leucine-rich repeat (LRR) domain in their extracellular domain and a Toll/interleukin (IL)-1 receptor (TIR) domain in their intracellular domain. All 13 members of the TLR-family in mice, except TLR3, associate with a common adaptor molecule, MyD88, through interaction of their intracellular TIR domains to trigger inflammatory responses (11,12). TLR3 and TLR4 utilize an alternative adaptor protein, TRIF, to induce type I IFN. TLR4 requires the association with LPS-binding protein (LBP), CD14 and MD2 to recognize LPS. Upon TLR4 ligation, the intracellular domain of TLR4 recruits TIRAP and MyD88 for MyD88 dependent signaling and TRAM bridges TRIF for MyD88-independent signaling (13,14).
The pathogenesis of NAFLD is associated with TLR-signaling (15). Therefore, we will discuss in the next paragraphs the TLRs (i.e., TLR2, TLR4 and TLR9) that received particular interest in relation to NAFLD.
Toll-like receptor 4 (TLR4)
TLR4 is a receptor for lipopolysaccharide (LPS), which is a cell component of Gram-negative bacteria and has received particular interest in relation to hepatic inflammation and fibrogenesis (13,16). During NAFLD progression, metabolic alterations can affect the gut microbiota and gut permeability, thereby elevating circulating levels of LPS in humans as well as animal models of NAFLD [high fat diet, methionine/choline-deficient diet (MCD) and choline-deficient amino acid-define (CDAA) diet] (17,18). In the liver, Kupffer cells are among the first to be hit by bacterial or sterile insults and contribute to the inflammatory response. Kupffer cells express TLR4 and binding of LPS to this receptor results in activation of NF-κB, MAPK, ERK1, p38, JNK and IRF3 triggering the production of pro-inflammatory cytokines and type I interferon. These pro-inflammatory stimuli contribute to enhanced hepatocyte damage, increased leukocyte infiltration and secretion of pro-fibrogenic cytokines such a TGF-β and PDGF, promoting the activation of hepatic stellate cells and the fibrogenic response (13,16). Moreover, inactivation of TLR4 in mice results in a marked attenuation of steatohepatitis induced by a methionine-/choline-deficient diet (19), and leads to a significant decrease in high fat- and fructose-induced hepatic steatosis (20). Therefore, LPS and gut permeability are highly associated with the development of NAFLD, and TLR4 signaling is important to maintain the balance of inflammatory and fibrogenic signaling in the liver. Blocking TLR4 signaling or inhibiting LPS release from the intestinal microbiota may therefore represent a feasible strategy for the prevention or treatment of NAFLD.
Toll-like receptor 2 (TLR2)
TLR2 recognizes a variety of ligands, including bacterial, fungal, viral and some endogenous substances. Among them, certain components of Gram-positive bacterial cell walls (i.e., peptidoglycan and lipoteichoic acid) are demonstrated to activate TLR2 (12). Since levels of Firmicutes, which are Gram-positive bacteria, are elevated during obesity, these ligands are rich in gut microbiota of obese subjects and are associated with NAFLD disease progression. Initial studies demonstrated that the absence of TLR2 signaling was protective in diet-induced NASH in mice (high fat and CDAA diet) (21-23). In contrast, TLR2 deficient mice upon MCD diet exhibit equivalent or more severe NASH as a result of increased TLR4 expression and consequently hypersensitivity to LPS (24,25). Therefore, the difference in gut microbiota may be the reason for the contrasting results in TLR2-signaling and demonstrates how important the microbiome is during NAFLD progression.
Toll-like receptor 9 (TLR9)
Activation of TLR9 signaling gained a lot of interest lately, as bacterial translocation plays an important role in the pathogenesis of NAFLD. DNA derived from intestinal bacteria contains high amounts of cytidine-phosphateguanosine (CpG) oligonucleotides and these motifs are ligands for TLR9. These ligands can directly activate DCs, macrophages and B cells, and they result in a strong T helper 1 response (26). In a murine NASH model, bacterial DNA was detected in blood after 22 weeks of CDAA diet feeding (27). These findings suggested that activation of TLR9 signaling plays an important role in NAFLD. In addition, TLR9 deficient mice show less steatosis, inflammation and fibrosis than control mice, which was associated with decreased IL-1β production by Kupffer cells (27). Furthermore, insulin resistance and weight gain induced by the CDAA diet were also suppressed in TLR9 deficient mice. Taken together, these data demonstrate that TLR9 signaling promotes NAFLD disease progression.
Altogether, TLR-signaling in the pathophysiology of NAFLD results in tissue damage that sustains itself through other endogenous TLR-mediated mechanisms.
NOD-like receptors (NLRs)
Intracellular PRRs, such as NLRs are involved in the innate immune response by recognizing certain PAMPs (i.e., products derived from bacteria, virus, fungus and protozoa) and DAMPs (i.e., ATP, crystals, hyaluronan, and uric acid). Several NLR family members play a role in the formation of intracellular multiprotein complexes called inflammasomes. These complexes consist of an NLR protein [absent in melanoma 2 (AIM2) in response to cytoplasmic double stranded DNA (dsDNA)], the adapter molecule ASC, and the effector pro-caspase 1. The inflammasome serves as a platform for activating the cysteine protease caspase-1 that in turn results in the maturation of pro-inflammatory cytokines, including IL-1β and IL-18, and the proteolytic inactivation of IL-33. Furthermore inflammasomes contribute to the regulation of cell survival and cell death (28). Activation of the inflammasome is a 2-step process in which the priming step (injury, infection, or sterile inflammation) induces inflammasome expression and the second step triggers functional inflammasome activation by an inflammasome activator (29,30).
Inflammasome activation has been implicated in several liver diseases, including NAFLD (30). It has been shown that saturated fatty acids represent an endogenous danger in the form of a first hit, thereby inducing sensitization to LPS-induced inflammasome activation and inflammatory injury. Moreover, hepatocytes exposed to saturated fatty acids release danger signals that trigger inflammasome activation in immune cells (31). Upregulation and activation of inflammasome components are observed in male mice following a long term high fat diet, but not in females. These gender differences were probably related to the length of the high fat diet and/or the estrogen status of female mice (32). Furthermore, the role of NLRP3 during NASH development was studied by placing gain of function tamoxifen-inducible Nlrp3 knock-in mice and loss of function Nlrp3 knockout mice on choline-deficient amino acid-defined (CDAA) diet. Nlrp3−/− mice were protected from long-term feeding CDAA-induced hepatomegaly, liver injury, infiltration of activated macrophages and liver fibrosis, while Nlrp3 knock-in mice showed severe liver inflammation, with increased infiltration of activated macrophages and early signs of liver fibrosis. These data were confirmed in patients with NAFLD, demonstrating elevated levels of NLRP3 inflammasome components compared to patients with non-NASH NAFLD (31,33). Moreover, levels of pro-IL1β mRNA in these NAFLD patients correlated with the expression of the fibrosis marker COL1A1, indicating the importance of NLRP3 inflammasome activation during fibrosis development in NAFLD (34).
Caspase 1 activation in Kupffer cells induces pro-inflammatory signaling and hepatic stellate cell activation, which are then responsible for collagen deposition and fibrosis during diet-induced NASH (35,36). In addition, hyperlipidemic Ldlr−/− mice transplanted with caspase1/11-deficient bone marrow cells showed less hepatic inflammation and cholesterol crystals inside Kupffer cells upon high cholesterol high fat diet feeding compared to wild type transplanted mice (37). In line with these data, it has been demonstrated that cholesterol crystallization within hepatocyte lipid droplets and aggregation and activation of Kupffer cells in crown-like structures around such droplets represent an important, novel mechanism for progression of simple steatosis to NASH in both humans and mice (38). Furthermore, Csak et al. demonstrated that both bone marrow-derived and non-bone marrow-derived cells contribute to inflammasome activation in a MyD88-dependent manner in dietary NASH. They demonstrated that AIM2 inflammasome expression and activation were further augmented by TLR9 ligands during NAFLD progression (39). Altogether, these data indicate that inflammasome activation during NASH development is maintaining the inflammatory response in the liver.
In contrast to the data mentioned above, Henao-Mejia et al. demonstrated that modulation of the intestinal microbiota through multiple inflammasome components is a critical determinant of NAFLD/NASH progression, as well as multiple other aspects of the metabolic syndrome such as weight gain and glucose homeostasis (40). The authors described that NLRP3 and NLRP6 inflammasomes and the effector protein IL-18 negatively regulate NAFLD/NASH progression via alterations in the gut microbiota. They demonstrated in different mouse models that hepatic steatosis as well as inflammation are exacerbated through influx of TLR4 and TLR9 agonists into the portal circulation, leading to enhanced hepatic TNF-α expression that drives NASH progression (40). Thus, altered interactions between the gut microbiota and the host may govern the rate of progression of multiple metabolic syndrome-associated abnormalities, highlighting the central role of the microbiota.
Gut-liver interactions and the impact of the microbiome on NAFLD progression
The inflammatory phenotype during NAFLD progression can be attributed to the innate immune system, which is the first line of defence against invading pathogens and is crucial for the overall survival of the host. The liver’s strategic location is optimal for the translation of physiological and pathological processes within the gastrointestinal tract into metabolic and immunologic outcomes. Therefore, the intestinal microbiota is a central component of hepatic pathophysiology. Under homeostatic conditions, the interaction between commensal micro-organisms and the hosts PRRs is necessary to locally maintain the intestinal microbial balance. In case of both early- and end-stage liver disease, alterations in the gut microbiota are important for the induction as well as the complications of the disease (41). Therefore, metabolic alterations during NAFLD progression can deregulate the innate immune signaling, thereby triggering bacterial translocation (17,18,42).
The role of gut-derived factors on NAFLD progression has just begun to be investigated. In humans, obesity is the most prevalent risk factor for NAFLD. The intestinal bacterial communities has been shown to be a critical modulator of body weight and body fat composition and correlate with multiple inflammatory and metabolic parameters as well as dietary habits in humans (15). Recent findings demonstrated that small intestinal bacterial outgrowth (SIBO) is a key determinant factor for NAFLD progression and is secondary to low intestinal motility. Furthermore, obese individuals have significantly increased levels of SIBO compared to healthy lean subjects, which is associated with increased gut permeability (18,43,44).
Therefore, these data indicate that bacterial translocation of certain bacterial communities might lead to portal endotoxemia and eventually hepatic injury. Most studies have shown that the levels of Firmicutes are increased whereas those of Bacteroidetes are decreased in obesity and metabolic disorders in humans as well as rodents. Therefore, an increased Firmicutes/Bacteroidetes ratio is a potential phenotype of obesity (15) and one may speculate that a larger amount of TLR2 ligands is delivered to the liver, as Firmicutes are Gram-positive bacteria. However, the exact contribution of Firmicutes and Bacteroidetes to the metabolic syndrome remains unknown (15). The development of obesity and NAFLD is also associated with a decrease in Akkermansia muciniphila, which are mucin-degrading bacteria residing in the mucus layer of the intestine (45,46). The proportion of Akkermansia muciniphila is inversely correlated with body weight in rodents and humans, and probiotic treatment can increase the abundance of these bacteria and improve metabolic parameters in obese mouse models. In addition, Akkermansia muciniphila treatment is able to reverse fat gain, serum LPS levels, gut barrier function and insulin resistance (47). Therefore, Akkermansia muciniphila can lead to thinner intestinal mucus layer and gut permeability, thereby allowing the leakage of bacterial components and influencing NAFLD progression.
Gut microbiota can also contribute to liver injury in patients with NAFLD by endogenous alcohol production. This endogenous alcohol can affect intestinal permeability, leading to endotoxemia and NAFLD progression. In children, the levels of Escherichia were significantly increased in NASH compared with those in obese control. Patients with NASH also had higher blood ethanol levels than the healthy and obese non-NASH subjects, suggesting a link between gut microbiota, endogenous alcohol and NAFLD progression. However, it is currently unclear whether an increase in Escherichia is a common mechanism of adult NASH (48).
Diet is a likely factor in altering gut microbiota composition and consequently playing a role in the observed dysbiosis during NAFLD progression. High fat diets alter gut microbiota composition by altering phyla ratios and promoting growth of Proteobacteria, which can lead to an increased pro-inflammatory potential of the microbiota (49). Next to the diets enriched with fat, fructose consumption has also greatly increased in recent years (50-52). Hepatic lipid accumulation and an altered microbiota composition were observed in mice placed on a high-fructose diet. Furthermore, it has been demonstrated that high-fructose diets result in rapidly reduced expression of tight junction proteins and therefore altering the gut barrier function. Consequently, expression of hepatic TLRs is elevated, thereby promoting the inflammatory response during high fructose consumption (53).
Changes in intestinal motility, the subsequent alteration of the microflora, decreased mucosal integrity and increased intestinal permeability are required for microbial products to translocate from the intestinal lumen to the extra-intestinal space, such as the liver. As a consequence of this defective barrier, elevated PAMP levels can often be detected in systemic circulation of diseased persons and further contribute to inflammation and fibrogenesis via PRR signaling (54).
In liver, Kupffer cells can respond to very low concentrations of microbial products such as LPS via activation of NF-κB and production of pro-inflammatory cytokines (55). This hyper-responsiveness of Kupffer cells to LPS is also observed in high fat diet induced steatosis in mice and is linked to TLR4 signaling and the upregulation of CD14 by a leptin-mediated signal (56). Other liver cells such as hepatic stellate cells, endothelial cells, biliary epithelial cells and hepatocytes can also respond to TLR-agonists, thereby triggering NF-κB signaling and fibrogenesis in the liver. These observations are also confirmed by other studies demonstrating that, in mice, diet-induced metabolic syndrome is absent in germ-free conditions (57). In addition, transfer of microbiota from steatotic mice into germfree mice promoted development of high fat diet-induced steatosis compared to germfree mice given the microbiota of non-steatotic mice (58,59). Such steatosis correlated with dysglycemia, suggesting that the altered microbiota was broadly promoting the metabolic syndrome. Therefore, a central hypothesis proposed by several researchers is that increased levels of PRR activation by gut microbiota play a central role in chronic inflammatory processes in the liver (53).
Altogether, characterization of the (specific) bacterial communities and their metabolites should be investigated in large cohorts of patients and in more detail regarding their impact on NAFLD disease progression.
Linking inflammation to obesity and metabolic consequences
Low grade chronic inflammation is fundamental in the progression of NAFLD and is a key feature of obesity. These inflammatory processes involve many components of the classical inflammatory response to pathogens and include systemic increases in circulating inflammatory cytokines and acute-phase proteins, recruitment of leukocytes to inflamed tissues, activation of tissue leukocytes and generation of reparative tissue responses like fibrosis (6,60). These innate immune responses demonstrate the importance of PRRs in activating important stress pathways and disrupting critical metabolic processes implicated in obesity (30). Activation of PRRs can lead to peripheral insulin resistance in the liver, muscle and adipose tissue, promote central leptin/insulin resistance and disrupt neuronal control of energy balance in the hypothalamus, impair insulin secretion in pancreatic islets and promote the pathogenesis of atherosclerosis in blood vessel walls.
Furthermore activated PRRs can affect gut microbiota and permeability, thereby triggering an influx of various microbiota-derived PAMPs into the circulation that activate their corresponding PRRs in many tissues (61). In addition, intestinal microbiota might contribute to NAFLD progression by modifying intestinal bile acids (62). Bile acids mediate communication between the liver and intestine by the absorption of dietary fats and vitamins and by acting as ligands for the nuclear receptor farnesoid X receptor (FXR) and the G-protein-coupled receptor TGR5 (54,63). Therefore, changes in the intestinal microbiota and enterohepatic circulation can influence NAFLD progression.
Immune cells are key players in metabolic homeostasis. They respond to metabolic stress and produce pro- and/or anti-inflammatory mediators to modulate metabolite programs. Immune cells start to infiltrate into the adipose tissue at the onset of weight gain and contribute to and perpetuate the inflammatory response, eventually leading to systemic insulin resistance and development of obesity (30). A similar effect is observed during NAFLD progression, with increases in the number of immune cells infiltrating in the liver and elevated cytokine levels (64). The inflammatory response during NAFLD is further supported by elevated levels of certain metabolites such as fatty acids, ceramides and cholesterol crystals, thereby affecting pathogen-sensing signaling pathways (e.g., TLRs, NLRs, JNK, NFκB) (5,65). Therefore, metabolic stress initiates a feed forward cycle of inflammatory responses, resulting in a state of unresolved chronic inflammation.
Future perspectives
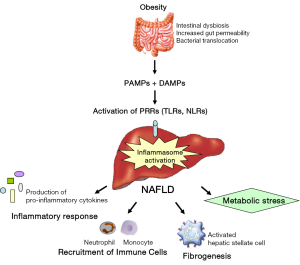
The liver is the principal metabolic regulator of the body. Therefore, alterations in the homeostatic state of host-microbial interactions in the gastrointestinal tract could potentially lead to severe metabolic and inflammatory pathologies in the liver. Accumulating data demonstrate that PRR signaling and gut microbiota are closely associated with NAFLD progression. Changes in intestinal motility, the subsequent alteration of the microflora, decreased mucosal integrity and increased intestinal permeability are required for microbial products to translocate from the intestinal lumen to the liver, thereby contributing to inflammation and fibrogenesis via PRR signaling (Figure 1). For a better understanding of the impact of the microbiome on the inflammatory response during NAFLD, characterization of the (specific) bacterial communities and their metabolites should be investigated in large cohorts of patients and in more detail. The ultimate goal is to restore eubiosis, thereby resulting in restoration of intestinal homeostasis and symbiosis. Therefore, these new insights could potentially lead to new therapeutic approaches for the prevention and/or treatment of NALFD.
Acknowledgements
This work was supported by a grant from the Alexander von Humboldt Foundation (3.3-BEL/1147960 STP) and the German Research Foundation (DFG: BI 1670/2-1 + TR 285/10-1; SFB/TRR57).
Disclosure: The authors declare no conflict of interest.
References
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836-46. [PubMed]
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010;5:145-71. [PubMed]
- Wree A, Broderick L, Canbay A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 2013;10:627-36. [PubMed]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415-45. [PubMed]
- Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014;28:637-53. [PubMed]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111-7. [PubMed]
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124-31. [PubMed]
- Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis 2010;28:162-8. [PubMed]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805-20. [PubMed]
- Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol 2009;21:10-6. [PubMed]
- Broering R, Lu M, Schlaak JF. Role of Toll-like receptors in liver health and disease. Clin Sci (Lond) 2011;121:415-26. [PubMed]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373-84. [PubMed]
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 2008;48:322-35. [PubMed]
- Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract 2010;2010.
- Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:7381-91. [PubMed]
- Pradere JP, Troeger JS, Dapito DH, et al. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis 2010;30:232-44. [PubMed]
- Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012;590:447-58. [PubMed]
- Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877-87. [PubMed]
- Csak T, Velayudham A, Hritz I, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol 2011;300:G433-41. [PubMed]
- Spruss A, Kanuri G, Wagnerberger S, et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009;50:1094-104. [PubMed]
- Ehses JA, Meier DT, Wueest S, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 2010;53:1795-806. [PubMed]
- Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J 2010;24:731-9. [PubMed]
- Miura K, Yang L, van Rooijen N, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013;57:577-89. [PubMed]
- Szabo G, Velayudham A, Romics L Jr, et al. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res 2005;29:140S-145S. [PubMed]
- Rivera CA, Gaskin L, Allman M, et al. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol 2010;10:52. [PubMed]
- Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int 2013;7:771-81. [PubMed]
- Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 2010;139:323-34.e7.
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519-50. [PubMed]
- Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature 2012;481:278-86. [PubMed]
- Bieghs V, Trautwein C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol 2013;34:446-52. [PubMed]
- Csak T, Ganz M, Pespisa J, et al. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011;54:133-44. [PubMed]
- Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol 2014;20:8525-34. [PubMed]
- Wree A, Eguchi A, McGeough MD, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014;59:898-910. [PubMed]
- Wree A, McGeough MD, Peña CA, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 2014;92:1069-82. [PubMed]
- Dixon LJ, Berk M, Thapaliya S, et al. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest 2012;92:713-23. [PubMed]
- Dixon LJ, Flask CA, Papouchado BG, et al. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One 2013;8:e56100. [PubMed]
- Hendrikx T, Bieghs V, Walenbergh SM, et al. Macrophage specific caspase-1/11 deficiency protects against cholesterol crystallization and hepatic inflammation in hyperlipidemic mice. PLoS One 2013;8:e78792. [PubMed]
- Ioannou GN, Haigh WG, Thorning D, et al. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res 2013;54:1326-34. [PubMed]
- Csak T, Pillai A, Ganz M, et al. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis. Liver Int 2014;34:1402-13. [PubMed]
- Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179-85. [PubMed]
- Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59-64. [PubMed]
- Henao-Mejia J, Elinav E, Thaiss CA, et al. Role of the intestinal microbiome in liver disease. J Autoimmun 2013;46:66-73. [PubMed]
- Sabaté JM, Jouët P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg 2008;18:371-7. [PubMed]
- Wigg AJ, Roberts-Thomson IC, Dymock RB, et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001;48:206-11. [PubMed]
- Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. ISME J 2012;6:1449-58. [PubMed]
- Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004;54:1469-76. [PubMed]
- Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014;63:727-35. [PubMed]
- Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601-9. [PubMed]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009;137:1716-24.e1-2.
- Tappy L, Lê KA. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin Res Hepatol Gastroenterol 2012;36:554-60. [PubMed]
- Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes Rev 2012;13:799-809. [PubMed]
- Wagnerberger S, Spruss A, Kanuri G, et al. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr 2012;107:1727-38. [PubMed]
- Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology 2014;59:328-39. [PubMed]
- Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513-24. [PubMed]
- Su GL, Klein RD, Aminlari A, et al. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 2000;31:932-6. [PubMed]
- Imajo K, Fujita K, Yoneda M, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab 2012;16:44-54. [PubMed]
- Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010;24:4948-59. [PubMed]
- Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013;62:1787-94. [PubMed]
- Gangarapu V, Yıldız K, Ince AT, et al. Role of gut microbiota: obesity and NAFLD. Turk J Gastroenterol 2014;25:133-40. [PubMed]
- Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012;249:218-38. [PubMed]
- Jin C, Flavell RA. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol 2013;132:287-94. [PubMed]
- Tanaka N, Matsubara T, Krausz KW, et al. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012;56:118-29. [PubMed]
- Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 2006;103:3920-5. [PubMed]
- Wouters K, van Gorp PJ, Bieghs V, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008;48:474-86. [PubMed]
- Radwan MM, Radwan BM, Nandipati KC, et al. Immunological and molecular basis of nonalcoholic steatohepatitis and nonalcoholic fatty liver disease. Expert Rev Clin Immunol 2013;9:727-38. [PubMed]


