Dietary saturated fatty acids reduce hepatic lipid accumulation but induce fibrotic change in alcohol-fed rats
Introduction
Both dietary fatty acids and alcohol play important roles in the pathogenesis of alcoholic liver disease (ALD). The roles of different types of dietary fats in ALD are supported by epidemiologic studies. Those studies indicated that susceptibility to alcohol is related to different types of fatty acids in the diet. Nanji and French demonstrated that the expected mortality from cirrhosis was correlated with the dietary fatty acid composition, and saturated fatty acids (SFAs) were protective against ALD (1). Several animal studies also indicated that SFAs reduced hepatotoxicity induced by alcohol consumption including fatty liver, fibrotic changes, and necrosis (2-8). Recently, You et al. demonstrated that the protective effects of SFAs against alcoholic fatty liver may occur via the sirtuins (SIRT)-1-sterol regulatory element-binding protein 1 (SREBP-1) histone H3 axis which suppresses expressions of genes encoding lipogenic enzymes and slows the synthesis of hepatic fatty acids (9,10). However, most of these studies used vegetable oils such as palm oil, cocoa butter, and medium-chain triglycerides as fat sources for the SFAs. There is little evidence about animal SFAs on lipid synthesis and metabolism in ALD.
Lard is the most common source of dietary fat in Taiwan. Taiwanese often use lard to fry vegetables and make some Chinese-style desserts. In addition, mortality from ALD is increasing, and the public health topic of chronic alcohol consumption is becoming important in Taiwan. Therefore, we used lard as a source of dietary fats to investigate its effects on ALD in rats fed an ethanol-containing diet.
Materials and methods
Animals
Male Wistar rats (BioLasco Taiwan, Taiwan) weighing about 160 g were used in this experiment. All rats were housed in individual stainless-steel cages in an animal room maintained at 22±2 °C with 50-70% humidity and a 12-h light-dark cycle. All rats were allowed free access to a standard rodent diet (LabDiet 5001 Rodent Diet; PMI Nutrition International, MO, USA) and water for 1 week during acclimation before the study. All procedures were approved by the Institutional Animal Care and Use Committee of Taipei Medical University.
Study protocol
After 1 week of acclimation, rats were assigned to groups based on their levels of activity of plasma aspartate transaminase (AST) and alanine transaminase (ALT), so there would be no significant differences between groups in their plasma AST and ALT activity levels. The four groups were as follows: an ethanol (E) group fed an ethanol liquid diet (containing 36% energy as fat) with a Ritcher drinking tube; a control (C) group pair-fed an isoenergetic diet without ethanol; an ethanol with high-SFA diet (EHS) group fed an ethanol-containing diet, which contained 90% lard and 10% soybean oil substitute for corn oil, olive oil and safflower oil in the diet of E group; and a control with high-fat diet (CHS) group fed an isoenergetic diet without ethanol, which contained lard and soybean oil as the fat source. Compositions of the experimental diets of all groups are given in Table 1. After 8 weeks of the experimental period, rats were anesthetized and sacrificed. Blood samples were collected in heparin-containing tubes and centrifuged (1,200 ×g for 15 min at 4 °C) to separate the plasma. All plasma samples were stored at −80 °C until being assayed. Liver tissues were rapidly excised and stored at −80 °C for further analysis.
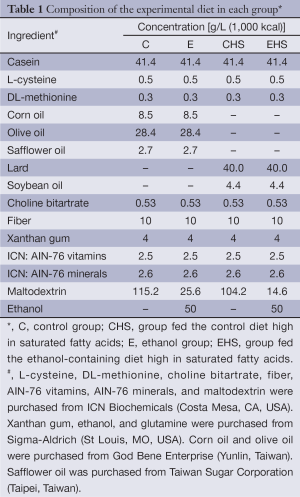
Full table
Measurements and analytical procedures
Liver function
Plasma AST and ALT activities were measured as indices of liver function using a colorimetrical method with an automatic analyzer, Beckman Synchron LXTM system (GMI, MN, USA). The system monitors the change in absorbance at 340 nm, and then calculates and expresses the AST and ALT activities.
Hepatic histopathology and plasma TGF-β1 concentration
Formalin-fixed liver tissues were stained with hematoxylin-eosin (H&E) and trichrome stains. A semiquantitative histological evaluation was carried out by a pathologist blinded to the treatment groups to assess the degree of tissue inflammation, fatty changes, necrosis of hepatocytes, and tumor burden. The grading ranged 0-4 where 0= absent, 1= trace, 2= mild, 3= moderate, and 4= severe.
The plasma TGF-β1 concentration was measured using a rat TGF-β1 kit (R&D Systems, MN, USA), and the optical density was read at 450 nm using a microplate reader (Molecular Devices, CA, USA).
Factors influencing lipogenesis and lipolysis
Plasma adiponectin concentration
The plasma adiponectin concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (AssayMax rat adiponectin ELISA kit, MO, USA). The optical density was read at 450 nm for all cytokines using a microplate reader (Molecular Devices, CA, USA).
Hepatic SIRT-1 expression
A Western blot analysis was performed to determine the expression of SIRT-1. Liver tissues were homogenized in 5 volumes of buffer (25 mM HEPES at pH 7.6, 300 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1% Triton-X, and 10% glycerol). After shaking for 10 min, the homogenate was centrifuged at 13,200 ×g for 40 min at 4 °C. The supernatant was centrifuged at 55,000 rpm for 60 min at 4 °C. The supernatant was separated by 10% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then proteins were electroblotted onto a polyvinylidene difluoride (PVDF) transfer membrane (Amersham Biosciences, UK). These blots were incubated with rabbit monoclonal anti-rat SIRT-1 (Aviva Systems Biology, CA, USA) and mouse anti-actin monoclonal antibodies (Chemicon International, CA, USA). Finally, the blot was treated with goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP) (Chemicon International, CA, USA), and specific bindings of antibodies were detected using a Western Lightning kit (PerkinElmer Lifesciences, MA, USA). Bands were quantified using Image-Pro Plus software (vers. 4.5.0.29; MD, USA).
Plasma non-esterified fatty acid (NEFA) levels
Plasma NEFAs were determined using a commercial kit (Randox Laboratories, UK). Ten microliters of sample or standard was added to 200 µL of reagent 1 (0.04 mol/L phosphate buffer, 3 mmol/L magnesium chloride, 0.3 U/mL acyl coenzyme A synthetase, 1.5 U/mL ascorbate oxidase, 0.9 mmol/L coenzyme A, 5.0 mmol/L ATP, and 1.5 mmol/L 4-aminoantipyrine), mixed well, and incubated for 5 min at 37 °C. Then, 400 µL of reagent 2 (0.3% w/v phenoxyethanol, 10.6 mmol/L maleimide, 10 U/mL acyl coenzyme A oxidase, 7.5 U/mL peroxidase, and 1.2 mmol/L TOOS) was added. After incubating the mixture at 37 °C for 5 min, NEFA concentrations were measured at 550 nm.
Hepatic peroxisome proliferator activated receptor (PPAR)-α expression
A Western blot analysis was performed to determine the expression of PPAR-α. Liver tissues (0.5 g) were homogenized in 1.5 mL RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, and 1% NP-40 at pH 7.5). After an ice bath for 30 min, the homogenate was centrifuged at 10,000 ×g for 10 min at 4 °C. The supernatant was separated by 10% SDS-PAGE. Then proteins were electroblotted onto a PVDF transfer membrane (Amersham Biosciences, UK). These blots were incubated with rabbit monoclonal anti-rat PPAR-α (Aviva Systems Biology, CA, USA) and mouse anti-actin monoclonal antibodies (Chemicon International, CA, USA). Finally, the blot was treated with goat anti-rabbit IgG-HRP (Chemicon International, CA, USA), and specific bindings of antibodies were detected using a Western Lightning kit (PerkinElmer Lifesciences, MA, USA). Bands were quantified using Image-Pro Plus software (vers. 4.5.0.29, MD, USA).
Oxidative stress
Hepatic reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio
Liver tissue (0.5 g) was homogenized in 1.5 mL ice cold buffer (50 mM Tris at pH 7.2, 150 mM NaCl, and 1% Triton-X) plus 0.1% of a protease inhibitor. The homogenate was then shaken on ice for 90 min. After shaking, the homogenates were centrifuged at 3,000 ×g and 4 °C for 15 min. The GSH/GSSG ratio, lipid peroxide concentration, and tumor necrosis factor-α (TNF-α) concentration of the supernatants were analyzed.
The GSH concentration was measured spectrophotometrically at 405 nm according to the method of Tietze (11), and the GSSG concentration was measured spectrophotometrically at 405 nm according to the method of Griffith (12). The ratio of GSH/GSSG was calculated as (GSH-2GSSG)/(GSSG).
Plasma and hepatic thiobarbituric acid (TBA)-reactive substances (TBARSs)
Lipid peroxidation was determined by measuring the concentration of TBARSs in the liver with minor modifications (13). Briefly, 20 mL of liver homogenates or plasma sample or 1,1,3,3-tetramethoxypropane (as the standard) was shaken with 800 mL of 0.22% H2SO4 in a 2-mL centrifuge tube. Then, 100 mL of 10% phosphotungstic acid and 200 mL of 0.67% TBA were added to the mixture, mixed well, and warmed for 60 min in a boiling water bath followed by rapid cooling. This was shaken into 600 mL of an n-butanol layer in a separation tube, and the n-butanol layer was measured fluorometrically (at respective excitation and emission wavelengths of 531 and 590 nm) using a Wallac Victor-2 1420 Multilabel Counter (PerkinElmer, MA, USA).
Hepatic microsomal CYP2E1 protein expression
Liver tissues were homogenized in 10 volumes of ice-cold buffer (0.25 M sucrose, 10 mM Tris-HCl, and 0.25 mM phenylmethylsulfonyl fluoride at pH 7.4). The homogenates were centrifuged at 17,000 ×g for 20 min at 4 °C. The supernatant was transferred to a new tube and centrifuged at 105,000 ×g for 60 min at 4 °C to separate the microsomes. The microsomal pellet was dissolved in 50 mM potassium phosphate buffer containing 1 mM EDTA and 1 mM DTT (pH 7.4). Microsomal proteins (30 µg) were separated by 10% SDS-PAGE. After separation, the proteins were electroblotted onto a PVDF transfer membrane (Amersham Biosciences, UK). These blots were then incubated with mouse monoclonal anti-rat CYP2E1 (Oxford Biomedical Research, MI, USA) and mouse anti-actin monoclonal (Chemicon International, CA, USA) antibodies. Finally, the blot was treated with goat anti-mouse IgG-HRP (Chemicon International, CA, USA) or goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology, CA, USA), and specific bindings of antibodies were detected using a Western Lightning kit (PerkinElmer Lifesciences, MA, USA). The bands were quantified using Image-Pro Plus 4.5 software analysis (vers. 4.5.0.29, MD, USA).
Inflammatory factors
Hepatic cytokine concentrations were measured using ELISA kits. TNF-α, interleukin (IL)-1β, IL-6, and IL-10 concentrations were respectively determined with a rat TNF-α/TNFSF1A kit, a rat IL-1β/IL-1F2 kit, a rat IL-6 kit, and a rat IL-10 kit (all from R&D Systems, MN, USA). The optical density was read at 450 nm for all cytokines using a microplate reader (Molecular Devices, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) followed by Fisher’s test was used to determine statistical differences among groups using SAS software vers. 8.0 (SAS Institute, Cary NC, USA). Statistical significance was assigned at the P<0.05 level.
Results
Energy and alcohol intake
The average energy intake in the four groups was as follows: C group (74.8±11.6 kcal/day), CHS group (75.1±10.6 kcal/day), E group (76.4±9.6 kcal/day), and EHS group (63.9±9.5 kcal/day). There were no differences among these groups in average energy intake. The average alcohol intake levels in the groups consuming ethanol were as follows: E group (3.8±0.5 g/day), and EHS group (3.2±0.5 g/day). No difference was observed between E and EHS groups in alcohol intake.
Body weight (BW) and relative liver weight
The initial BW, final BW, and relative liver weight of rats in each group are listed in Table 2. There were no significant differences in initial BWs of rats among all groups, however, EHS group showed the lowest BW among all groups. In addition, the relative liver weights of E and EHS groups were significantly higher than that of C group (P<0.05).

Full table
Liver function
Plasma AST and ALT activities of each group are shown in Table 3. The plasma AST activities of E and EHS groups were significantly higher than that of C group after the 8-week feeding (P<0.05). The plasma ALT activity of E group showed an increasing trend compared to C group. Otherwise, the plasma ALT activity of EHS group was significantly higher than those of C and CHS groups (P<0.05).

Full table
Hepatic histopathology and plasma TGF-β1 concentration
Hepatic histopathology scores are presented in Table 4. As shown in Table 4, inflammatory cell infiltration, cell degeneration and necrosis, and fatty changes were observed in E group. On the other hand, inflammatory cell infiltration and fatty changes in EHS group were lower than those in E group; however, significantly higher hypertrophy of hepatocytes, bile duct hyperplasia, and fibrosis were only found in EHS group (P<0.05). As shown in Figure 1A, fatty changes were found in E group; in addition, collagen generation was observed in CHS, E, and EHS groups (Figure 1B). Plasma TGF-β1 concentrations in the 8th week of the experiment are given in Table 5. A significantly higher plasma TGF-β1 concentration was observed in EHS group (P<0.05).
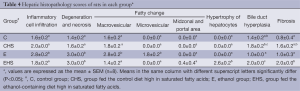
Full table

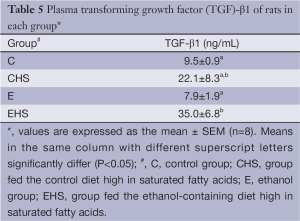
Full table
Factors influencing lipogenesis and lipolysis
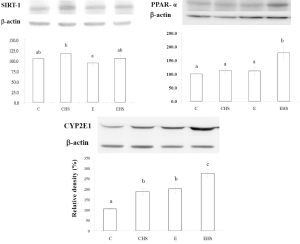
As shown in Table 6, a significantly lowest plasma adiponectin concentration was found in the E group (P<0.05). Plasma adiponectin in EHS group was lower than that in C group, but higher than that in E group (P<0.05). The plasma free fatty acid levels of each group are given in Table 6. It indicated that plasma free fatty acid levels in E and EHS groups were significantly lower than that of C group (P<0.05). As shown in Figure 2, lower hepatic SIRT-1 expression was found in E group, but it failed to reach statistical significance. Moreover, there was no significant difference in hepatic SIRT-1 expression between E and EHS groups. As shown in Figure 2, hepatic PPAR-α in EHS group was significantly higher than those of the other three groups (P<0.05).
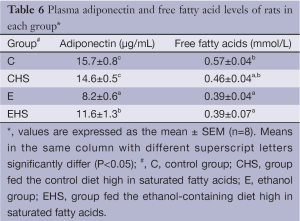
Full table
Oxidative stress
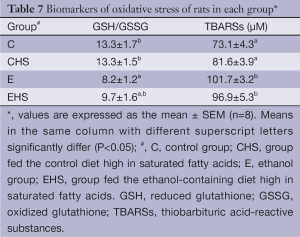
Biomarkers of oxidative stress of rats in each group are presented in Table 7. E group showed a significantly lower hepatic GSH/GSSG ratio (P<0.05); however, the hepatic GSH/GSSG ratio between E and EHS groups showed no significant difference. Hepatic TBARS concentrations in E and EHS groups were significantly higher than that in C group (P<0.05). Moreover, CYP2E1 expression is shown in Figure 2. CYP2E1 expression of E group was significantly higher than that of C group (P<0.05). In addition, EHS group showed the highest CYP 2E1 expression among all groups (P<0.05).
Full table
Inflammatory factors
Hepatic cytokines in each group are given in Table 8. E group showed significantly higher hepatic TNF-α, IL-1β, IL-6, and IL-10 concentrations than those of rats in C group. Hepatic TNF-α and IL-1β concentrations in EHS group did not significantly differ compared to those of E group; however, hepatic IL-6 and IL-10 in EHS group were significantly lower than those of E group (P<0.05).

Full table
Discussion
In this study, we used lard as a source of dietary fat to investigate its effect on ALD in rats fed an ethanol-containing diet for 8 weeks.
BW, liver weight, and alcohol intake
The average energy intake was similar in all groups. The alcohol intake volume also showed no difference in the alcohol-fed groups. However, significantly higher relative liver weights in E and EHS groups indicated that hepatomegaly had occurred in both groups after 8 weeks of alcohol exposure (Table 2). Alcohol intake was reported to increase the relative liver weight in rats (14). Moreover, a previous study showed that isocaloric substitution of carbohydrates by ethanol resulted in significantly lower weight gain but a higher relative liver weight despite similar energy intake levels (15). Our findings indicate that alcohol intake accompanied with high dietary SFAs produced the lowest final BW even under similar energy consumption, but showed no effect on hepatomegaly induced by chronic alcohol feeding.
Liver function and hepatic histopathology
AST and ALT activities are markers of liver damage as opposed to alcohol misuse (16). Both transaminases are found in hepatocytes, but AST is also found in skeletal and myocardial cells. In alcohol-related liver damage, AST is elevated more than ALT, at least in part as a reflection of alcohol-related skeletal damage (17). Therefore, to evaluate liver damage caused by chronic ethanol feeding, plasma AST and ALT activities were determined in the present study. Increased plasma AST and ALT activities in rats fed an ethanol-containing diet after the 8-week feeding revealed liver injury in both groups (Table 3). In E group, inflammatory cell infiltration and fat accumulation were observed from results of the histopathology examinations (Table 4). However, rats fed alcohol and high saturated fats had slight fatty changes and inflammation but significantly severe hypertrophy of hepatocytes and fibrosis in the liver (Table 4). These results indicated that liver injury was present in E and EHS groups, and the type of liver injury differed between the E and EHS groups.
Factors influencing lipogenesis and lipolysis
Alcohol is thought to cause fatty liver by altering the hepatic NADH/NAD+ ratio, that is, inhibiting fatty acid oxidation and stimulating lipogenesis (18,19). More recent studies indicated that alcohol intake may influence the activity of PPAR-α, SREBP-1, and AMP-dependent protein kinase (AMPK) directly or by way of effects on adiponectin (20-23). Adiponectin, a hormone largely secreted by adipocytes, has two receptors, receptors 1 (AdipoR1) and 2 (AdipoR2). AdipoR1 is expressed in various tissues with a relatively high level in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver. Adiponectin protects against ALD by decreasing inflammation and lipogenesis; however, previous studies showed that adiponectin was lower in ethanol-fed rats and mice (24,25). You et al. also indicated that inhibition of SIRT-1 activity was associated with an increase in the acetylated active nuclear form of SREBP-1c in livers of ethanol-fed mice (21). Moreover, activated PPAR-α induces the expression of genes involved in mitochondrial and peroxisomal free fatty acid β-oxidation. PPAR-α also inhibits the lipogenic pathway by inducing malonyl CoA, a precursor of fatty acid synthesis (26). In vivo experiments on ethanol-fed rodents reported animal-specific differences in the effects of ethanol on hepatic PPAR-α protein levels. In SV/129 mice and in rats, ethanol administration decreased PPAR-α protein levels (27). On the other hand, PPAR-α protein did not change in ethanol-fed C57BL/6J mice; however PPAR-α binding to DNA was significantly impaired and some PPAR-α target genes were downregulated (28). However, we found that the plasma adiponectin level decreased, but SIRT-1 and PPAR-α expressions exhibited no changes in E group in this study (Figure 2). Although hepatic lipid accumulation was observed in rats fed ethanol, upstream factors related to lipogenesis and lipolysis, such as SIRT-1 and PPAR-α expressions, were not transformed in this study. Because it has been shown previously that chronic alcohol intake affected SIRT-1 activity (21), not protein itself, we should confirm the activities of these factors in a future study.
On the other hand, the increased expressions of adiponectin and PPAR-α in EHS group indicated that high dietary SFAs improved the β-oxidation of fatty acids in the liver via upregulation of the adiponectin-PPAR-α pathway, and then inhibited hepatic accumulation induced by chronic alcohol intake (Table 6, Figure 2). Our results are similar to those of a previous study which showed that a high-saturated-fat diet increased β-oxidation of fatty acids in the liver against alcoholic fatty changes (29). We also found that plasma free fatty acids decreased in both groups E and ES (Table 6), so we supposed the lower lipogenic response induced by SFAs occurred in liver tissues, not in adipose tissues.
In addition, PPAR-α also regulates a number of other biological processes such as cell proliferation, apoptosis, inflammation, oxidative stress, and hepatocarcinogenesis (30,31). In our study, EHS group showed significantly higher hepatic PPAR-α protein expression than did the other three groups (Figure 2), and we suggest that the higher hepatic PPAR-α protein expression in EHS group may have contributed to fewer fatty changes; however, it may also indicate possible hepatocarcinogenesis in this group.
Hepatic fibrosis
We also observed a higher plasma TGF-β1 level in the EHS group in our study. This result matches results of the hepatic histological analysis (Table 4, Figure 1). Carmiel-Haggai et al. showed that high saturated fat may cause hepatic fibrosis via increasing TGF-β (32). In addition to fibrosis, TGF-β also regulates chronic liver disease by contributing to all stages of disease progression from initial liver injury through inflammation and fibrosis to cirrhosis and hepatocellular carcinoma (33). Our data showed that chronic ethanol feeding together with a high-saturated-fat diet induced significantly higher plasma TGF-β concentrations; this indicated that hepatocellular carcinoma may develop in this group.
Oxidative stress
Oxidative stress is one of the mechanisms of alcoholic liver injury. As shown in Table 7, oxidative stress was found in rats after 8 weeks of alcohol exposure (group E); however, combining ethanol with saturated fats did not alter the hepatic GSH/GSSG ratio or TBARS concentration. This indicates that saturated fats, especially lard, did not change oxidative stress at least in this ethanol-exposure animal model. On the other hand, overexpression of hepatic CYP2E1 leads to ROS generation and highly reactive free radicals including superoxide anions and hydroxyl radicals which then result in oxidative stress in alcoholic liver injury (34,35). As shown in Figure 2, hepatic CYP2E1 expression increased in E group. Surprisingly, regardless of whether ingesting alcohol or not, high dietary SFAs increased hepatic CYP2E1 expression, and the highest level was observed in EHS group. Cytochrome P450 2E1 is involved in the microsomal ethanol-oxidizing system and is induced by heavy ethanol use. Morgan et al. stated that CYP2E1 can contribute to the development of hepatocarcinogenesis in two ways (36). First, CYP2E1 produces ROS that can lead to cell death and/or DNA damage and cancer initiation (37). Second, CYP2E1 oxidizes xenobiotics, including the carcinogens dimethylnitrosamine, aflatoxin, vinyl chloride, and dimethylhydrazine (38). Thus, even though oxidative stress, including lipid peroxidation and antioxidative substrate levels, was not increased by the high dietary SFA intake, CPY2E1 overexpression induced by the high dietary SFA intake still is a risk factor for hepatocarcinogenesis.
Proinflammatory and anti-inflammatory cytokines
Alcohol consumption can promote bacterial overgrowth, which may cause the accumulation of endotoxin. In addition, alcohol-induced gut barrier disruption leads to increased intestinal permeability, and excess amounts of endotoxin may transfer from the intestines to the liver and the general circulation and contribute to an inflammatory response (39,40). An increasing circulating endotoxin level induced by chronic alcohol intake activates innate immunity via stimulation of Kupffer cells to produce proinflammatory cytokines, such as TNF-α and IL-1β, that cause liver injury (41-44). In the present study, we observed that TNF-α and IL-1β levels significantly increased in E group compared to C group (Table 8). These results are consistent with those of previous studies (45-48). On the other hand, compared to E group, TNF-α and IL-1β levels in EHS group did not change. Thus, these results suggest that alcohol intake accompanied by high SFAs did not improve the production of proinflammatory cytokines.
In addition, activation of innate immunity also stimulates Kupffer cells to produce hepatoprotective cytokines (e.g., IL-6) and anti-inflammatory cytokines (e.g., IL-10 and adiponectin) in ALD (49,50). Recent studies suggested that both IL-6 and IL-10 play compensatory roles in protecting against ALD. Elevated IL-6 stimulates STAT3 activation in hepatocytes and upregulates the expression of antiapoptotic genes (e.g., Bcl-2 and Bcl-xL), antioxidative genes (e.g., metallothioneins 1 and 2), and mitochondrial DNA repair genes (e.g., OGG-1 and Neil 1) and downregulates lipogenic genes (e.g., SREBP-1) (51). A mice model of ALD demonstrated that IL-6-deficient mice were more susceptible to alcohol-induced hepatic steatosis, apoptosis, and mitochondrial DNA damage compared to wild-type animals (52). Thus, elevated IL-6 acts in a protective role in the progression of ALD by ameliorating steatosis and promoting liver regeneration (51,52). On the other hand, IL-10 is a well-documented anti-inflammatory cytokine. It was reported that IL-10-deficient mice exhibit increased liver inflammation and less steatosis after being fed alcohol or a high-fat diet (53). Those papers also proposed that IL-10 plays a dual role in regulating alcoholic fatty liver and liver injury (54). First, IL-10 inhibits LPS, TNF-α and complements activation, thereby reducing steatosis and liver damage. Second, IL-10 blocks IL-6 production, thereby reducing liver regeneration and enhancing steatosis and liver damage (54). In our study, hepatic IL-6 and IL-10 levels significantly increased in rats fed an ethanol-containing diet (E group). This result is consistent with previous animal studies of ALD. However, when dietary fatty acids of the ethanol-containing diet were high in SFAs, hepatic IL-6 and IL-10 levels significantly decreased in rats of EHS group. Thus, we surmised that high-dietary SFAs may inhibit increases in hepatoprotective and anti-inflammatory cytokines induced by ethanol feeding such as IL-6 and IL-10 in rats.
Conclusions
Chronic ethanol feeding for 8 weeks caused fatty changes and hepatic inflammation in rat livers. In addition, combining alcohol and saturated fats led to less fatty changes in rat livers; however, hepatic fibrosis was observed. We assumed that SFAs may improve hepatic fatty acid oxidation via increasing the expression of PPAR-α to inhibit hepatic fat accumulation, and increase the plasma TGF-β level to induce hepatic fibrosis. Moreover, chronic ethanol exposure together with saturated fats may contribute to decreased IL-6 and IL-10 levels which protect against ALD.
Acknowledgements
This study was supported by a grant from Nation Sciences Council, Taiwan (101-2918-I-038-001) to Suh-Ching Yang.
Disclosure: The authors declare no conflict of interest.
Ethical Statement: All procedures were approved by the Institutional Animal Care and Use Committee of Taipei Medical University.
References
- Nanji AA, French SW. Dietary factors and alcoholic cirrhosis. Alcohol Clin Exp Res 1986;10:271-3. [PubMed]
- Mezey E. Dietary fat and alcoholic liver disease. Hepatology 1998;28:901-5. [PubMed]
- Nanji AA, Sadrzadeh SM, Yang EK, et al. Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology 1995;109:547-54. [PubMed]
- Nanji AA, Yang EK, Fogt F, et al. Medium chain triglycerides and vitamin E reduce the severity of established experimental alcoholic liver disease. J Pharmacol Exp Ther 1996;277:1694-700. [PubMed]
- Nanji AA, Jokelainen K, Tipoe GL, et al. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther 2001;299:638-44. [PubMed]
- Nanji AA, Zakim D, Rahemtulla A, et al. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology 1997;26:1538-45. [PubMed]
- Ronis MJ, Korourian S, Zipperman M, et al. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr 2004;134:904-12. [PubMed]
- Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol 2004;34:21-5. [PubMed]
- You M, Cao Q, Liang X, et al. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr 2008;138:497-501. [PubMed]
- You M, Liang X, Ajmo JM, et al. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 2008;294:G892-8. [PubMed]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969;27:502-22. [PubMed]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 1980;106:207-12. [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8. [PubMed]
- Baumgardner JN, Shankar K, Korourian S, et al. Undernutrition enhances alcohol-induced hepatocyte proliferation in the liver of rats fed via total enteral nutrition. Am J Physiol Gastrointest Liver Physiol 2007;293:G355-64. [PubMed]
- Lin WT, Huang CC, Lin TJ, et al. Effects of beta-carotene on antioxidant status in rats with chronic alcohol consumption. Cell Biochem Funct 2009;27:344-50. [PubMed]
- Gordon H. Detection of alcoholic liver disease. World J Gastroenterol 2001;7:297-302. [PubMed]
- Conigrave KM, Saunders JB, Whitfield JB. Diagnostic tests for alcohol consumption. Alcohol Alcohol 1995;30:13-26. [PubMed]
- French SW. Nutrition in the pathogenesis of alcoholic liver disease. Alcohol Alcohol 1993;28:97-109. [PubMed]
- Lieber CS, DeCarli LM. Hepatotoxicity of ethanol. J Hepatol 1991;12:394-401. [PubMed]
- Xu A, Wang Y, Keshaw H, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003;112:91-100. [PubMed]
- You M, Fischer M, Deeg MA, et al. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 2002;277:29342-7. [PubMed]
- Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167-74. [PubMed]
- Galli A, Pinaire J, Fischer M, et al. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem 2001;276:68-75. [PubMed]
- Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 2008;60:790-7. [PubMed]
- Rasineni K, Casey CA. Molecular mechanism of alcoholic fatty liver. Indian J Pharmacol 2012;44:299-303. [PubMed]
- Lee TD, Sadda MR, Mendler MH, et al. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res 2004;28:173-81. [PubMed]
- Nanji AA, Dannenberg AJ, Jokelainen K, et al. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther 2004;310:417-24. [PubMed]
- Fischer M, You M, Matsumoto M, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 2003;278:27997-8004. [PubMed]
- Aoun M, Feillet-Coudray C, Fouret G, et al. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: effect of different nutritional lipid patterns. Br J Nutr 2012;107:647-59. [PubMed]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999;20:649-88. [PubMed]
- Peters JM, Cattley RC, Gonzalez FJ. Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis 1997;18:2029-33. [PubMed]
- Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J 2005;19:136-8. [PubMed]
- Meindl-Beinker NM, Matsuzaki K, Dooley S. TGF-β signaling in onset and progression of hepatocellular carcinoma. Dig Dis 2012;30:514-23. [PubMed]
- Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci 2007;81:177-87. [PubMed]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 2008;44:723-38. [PubMed]
- Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology 2004;127:S87-96. [PubMed]
- Nair J, Sone H, Nagao M, et al. Copper-dependent formation of miscoding etheno-DNA adducts in the liver of Long Evans cinnamon (LEC) rats developing hereditary hepatitis and hepatocellular carcinoma. Cancer Res 1996;56:1267-71. [PubMed]
- Seitz HK, Osswald B. Effect of ethanol on procarcinogen activation. In: Watson RR, editor. Alcohol and Cancer. Boca Raton, FL: CRC Press, 1992:55-72.
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res 2005;29:166S-71S. [PubMed]
- Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 2008;42:349-61. [PubMed]
- Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882-90. [PubMed]
- Gao B, Seki E, Brenner DA, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2011;300:G516-25. [PubMed]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009;50:638-44. [PubMed]
- Szabo G, Mandrekar P, Petrasek J, et al. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res 2011;35:782-6. [PubMed]
- Lecleire S, Hassan A, Marion-Letellier R, et al. Combined glutamine and arginine decrease proinflammatory cytokine production by biopsies from Crohn’s patients in association with changes in nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways. J Nutr 2008;138:2481-6. [PubMed]
- Thurman RG, Bradford BU, Iimuro Y, et al. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci 1999;4:e42-6. [PubMed]
- Chen YL, Yang SS, Peng HC, et al. Folate and vitamin B12 improved alcohol-induced hyperhomocysteinemia in rats. Nutrition 2011;27:1034-9. [PubMed]
- Peng HC, Chen YL, Chen JR, et al. Effects of glutamine administration on inflammatory responses in chronic ethanol-fed rats. J Nutr Biochem 2011;22:282-8. [PubMed]
- Mandal P, Park PH, McMullen MR, et al. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology 2010;51:1420-9. [PubMed]
- Petrasek J, Dolganiuc A, Csak T, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 2011;53:649-60. [PubMed]
- Horiguchi N, Wang L, Mukhopadhyay P, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology 2008;134:1148-58. [PubMed]
- El-Assal O, Hong F, Kim WH, et al. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol 2004;1:205-11. [PubMed]
- Miller AM, Wang H, Bertola A, et al. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology 2011;54:846-56. [PubMed]
- Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol 2012;27 Suppl 2:89-93. [PubMed]


