The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a significant cause of chronic liver disease and its prevalence is increasing worldwide. NAFLD is directly associated with the metabolic syndrome and obesity (1-3) and it is estimated that NAFLD affects approximately 90% of morbidly obese patients in the United States (4). In addition, to metabolic syndrome and obesity, insuline resistance and diabetes mellitus (DM) type 2 have been established as risk factors for NAFLD (5-10).
NAFLD is subdivided into nonalcoholic fatty liver (NAFL), in which hepatic steatosis is observed without significant inflammation and nonalcoholic steatohepatitis (NASH), in which hepatic steatosis is associated with inflammation. NAFLD, in particular the more aggressive form NASH, is increasingly observed as a cause of end stage liver disease and hepatocellular carcinoma (HCC), the most deleterious complication of cirrhosis. Obesity, DM and advanced fibrosis increase the risk of NASH progressing to cirrhosis and subsequently to HCC, however, the causal link between NASH and the development of HCC is still largely unclear.
Oxidative stress is considered an important factor in the pathogenesis of cancer including HCC (11) and reactive oxygen species (ROS) with consecutive DNA damage are induced by inflammatory processes. For example various cytokines can induce nitric oxide synthetase (iNOS), xantine oxidase (XO), myeloperoxidase (MPO) and NADPH oxidase (12,13). ROS can also be generated through other mechanisms, such as the induction of cytochrome P450 2E1 (CYP2E1), as demonstrated in the context of alcohol consumption (14,15).
ROS can react with polyunsaturated fatty acids derived from membrane phospholipids resulting in the production of reactive aldehydes as lipid oxidation (LPO) byproducts. The most abundant LPO byproducts are 4-hydroxynonenal (4 HNE) and malondialdehyde (MDA). These LPO byproducts react with DNA either directly or through bifunctional intermediates to form various mutagenic exocyclic etheno-DNA adducts. For example, LPO byproducts derived from y-linoleic acid, such as 4 HNE, react with the DNA bases A,C and G to yield inter alia the unsubstituted etheno-DNA adducts 1,N6-etheno-2'-deoxyadenosine (εdA), 3,N4-etheno-2'-deoxycytidine (εdC), 1,N2-etheno-2'-deoxyguanosine (1,N2εdG), and N2,3-etheno-2'-deoxyguanosine (N2,3εdG). In addition, DNA can also be modified directly by ROS to 8-nitrodG and 8-Oxo-dG (16).
Exocyclic etheno-DNA adducts are chemically stable and exhibit strong mutagenic properties, resulting in various types of base pair substitution mutations and other types of genetic damage in all organsims that have been tested so far (17,18). exocyclic etheno-DNA adducts can lead to to AT GC transversion and AT TA and AT CG transitions (19,20). Incorporation of a single exocyclic etheno-DNA adducts in either DNA strand of HeLa cells showed a similar miscoding frequency and was more mutagenic than 8-oxo-dG (21). In addition, individual etheno adducts are poorly repaired in certain cells and tissues, which further increases their biological relevance (22). Importantly, exocyclic etheno-DNA adducts are preferably formed in codon 249 of TP 53, resulting in inactivation of the tumor suppressor p53 and secondary cellular resistance to apoptosis and growth advantage (23). Formation of εdA and εdC in vivo by vinyl chloride, a confirmed human liver carcinogen, further supports the concept that etheno-DNA adducts play a causal role in the initiation and progression of HCC (24).
The aim of our study therefore was to investigate, if highly carcinogenic exocyclic etheno-DNA adducts are formed in livers of patients with NAFLD and if the generation of exocyclic etheno-DNA adducts correlate with the induction of CYP2E1, as it has been demonstrated in alcoholic liver disease (ALD).
Methods
Human liver species and metabolic data
Human liver fine-needle biopsy samples were obtained from 39 patients with NAFLD, 29 with NASH, 10 with NAFL, for diagnostic purposes at Salem Medical Center, University of Heidelberg, Germany. The liver specimens were fixed in formaldehyde for further testing.
Histologically normal liver sections from three healthy adult subjects were analysed as controls for exocyclic etheno-DNA adducts background staining intensity. These biopsies were originally taken to rule out hepatic tumours. The study was approved in accordance with the declaration of Helsinki by the Ethical Committee of the University of Heidelberg, Germany. All biopsies were assessed by histopathologists experienced in liver pathology and diagnosed as NASH or NAFL. Both investigators were blinded for immunohistopathology examinations related to the present study. Hepatic steatosis, inflammation and fibrosis were assessed using the scoring system of Kleiner et al. (25).
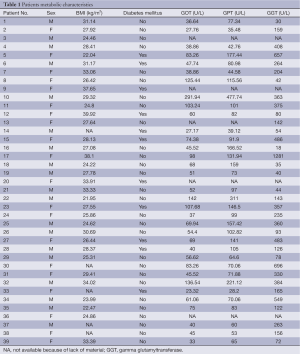
BMI was calculated as the individual’s body mass in kilogramm divided by the square of their height in meter. Eleven patients were treated for DM, aspartate aminotransferase (AST), alaninamino transferase (ALT) and gamma glutamyltransferase (GGT) were determined using standards procedures.
Immunohistochemical detection of etheno-DNA adducts
Staining was performed on liver tissue sections using the method developed in our laboratory (26-29). Paraffin-fixed slides were dipped in phosphate buffered saline (PBS) for 10 min and then placed in 0.3% H2O2 in absolute methanol for 10 min to quench endogenous peroxidase. Slides were incubated with proteinase K (20 mg/mL) (Roche, Mannheim, Germany) in double distilled H2O at room temperature for 10 min to remove histone and non-histone proteins from DNA increasing antibody accessibility. After washing with PBS, slides were treated with 20 µg/mL RNase (Roche, Mannheim, Germany) (heated for 10 min at 80 °C to inactivate DNase) at 37 °C for 1 hour to prevent antibody binding to RNA adducts and then washed in PBS. To denature DNA, cells were treated with 4N HCI for 5 min at room temperature and subsequently rinsed in double distilled water and PBS. The pH was neutralized with 50 mM Trisbase buffer, pH 7.4, for 5 min at room temperature. Non-specific binding sites were blocked with 8% bovine serum albumin (BSA), 2% normal horse serum, 0.05% Tween and 0.05% Triton X-100 for 20 min at 37 °C. Slides were incubated at 4 °C overnight with the primary monoclonal antibody EM-A-1 against εdA (provided by Drs. P. Lorenz and M. Rajewksy, University of Essen, Essen, Germany), at a dilution of 1:20 and 2% normal horse serum to block nonspecific binding. After washing with PBS, the antibody detection was performed using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s protocol [incubation with secondary antibody: horse anti-mouse IgG (H + L) 1:400 for 40 min at room temperature]. Diaminobenzidine (DAB) was used as a chromogen to visualize the reaction. The reaction was stopped after 5 min with H2O. Slides were counterstained with 4',6'-diamidino-2-phenylindole and mounted with Roti-Histokitt II (Carl Roth, Karlsruhe, Germany). All slides were subjected to the same standard conditions. Negative controls were performed by omitting the primary antibody.
Immunohistochemical staining of CYP2E1 and protein bound 4 HNE
Paraffin-embedded liver biopsy samples were cut into six micrometer sections and placed on 3-aminopropyl-triethoxysilan-coated glass slides. Sections were treated with 0.5% H2O2 in absolute methanol for 10 min to quench endogenous peroxidase activity. Thereafter, sections were incubated at room temperature for 2 hours with the primary antibody (rabbit anti-human CYP2E1, 1:400, Chemicon, Hofheim, Germany) or with rabbit anti-4 HNE (1:250, Alexis, Lörrach, Germany) and 5% normal goat serum to block nonspecific binding. Vectastain Elite ABC (Vector Laboratories; Burlingame, CA, USA) was used for detection according to the manufacturer’s protocol. Staining was developed by incubating the sections for 5 min in diaminobezidine (DAB). Sections were counterstained with hematoxylin and mounted with Roti-Histokitt II (Carl Roth, Karlsruhe, Germany). Negative controls were performed by omitting the primary antibody.
Imaging and semi-quantitative analyses of etheno-DNA adducts, CYP2E1 expression and protein-bound 4 HNE
After immunohistochemical staining for εdA, CYP2E1 and 4 HNE, the blinded slides were independently scored by a second investigator. Representative pictures were taken at a magnification of 100×/400× with a SPOT FLEX system (Model 15.2 64 Mp, shifting pixel, DIAGNOSTIC Instruments, Inc., Sterling Heights, Michigan, USA, SPOT VERSION 4.6.4.69) and analyzed using Image J software (U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/).
Staining intensity for CYP2E1 and 4 HNE was assessed according to the scale devised by Tsutsumi et al. (30), whereby 3+, 2+, 1+ and 0 denote intense, moderate, slight and no specific immunostaining, respectively. Staining intensity of εdA was estimated and recorded as 1 to 4. In addition, the frequency of nuclei positively stained for εdA was calculated as % of stained cell nuclei over a total number of cells counted. Finally, an intensity score (staining intensity x% of stained cell nuclei) was calculated.
Statistics
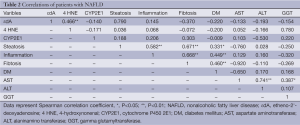
Spearman rank correlation was calculated to examine possible correlation between characteristics. A value of P<0.05 was used as level of significance.
Results
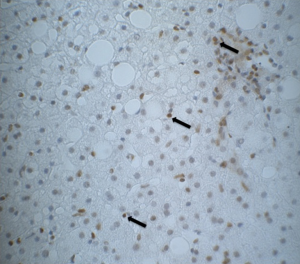
Table 1 shows the metabolic characteristic of our patients. In order to investigate the association of steatosis with DM we correlated the degree of steatosis in our liver biopsies with the presence of DM in our patients. As shown in Table 2, we found a significant positive correlation between the degree of hepatocellular steatosis and the presence of DM. In addition, hepatic steatosis correlated significantly with the degree of lobular inflammation and fibrosis. We also found a significant correlation between DM and lobular inflammation, as well as DM and fibrosis. Further, we analyzed CYP2E1, 4 HNE and εdA content in liver biopsies from our patients with NAFLD using immunhistochemistry. εdA was detected in a broad range of intensity (Figure 1), but unlike in ALD εdA did not correlate significantly with CYP2E1. However, we found a significant correlation between 4 HNE and εdA (Table 2).
Full table
Full table
Discussion
Hepatic steatosis is a manifestation of triglyceride accumulation in hepatocytes, mostly derived from fatty acids stored in adipose tissue and fatty acids synthesized by hepatocytes through de novo lipogenesis. Insulin resistance with consecutive increase of lipolysis in adipose tissue and excess importation of free fatty acid (FFA) to the liver is considered a major factor in the pathogenesis of hepatic steatosis. If lipid accumulation overwhelms the capacity of protective oxidative metabolic pathways in the liver, inflammatory cytokines may subsequently be induced (31), resulting in progression of NAFL to NASH. Consistent with this, we found a significant correlation between the presence of DM and the degree of steatosis and inflammation. Further we found a significant correlation between the degree of hepatocellular steatosis and lobular inflammation.
The most important finding of our study, however, is the observation of highly carcinogenic exocyclic etheno DNA lesions in the liver of patient with NAFLD. Previous studies had shown increased levels of carcinogenic etheno-DNA adducts that are formed by the reaction with the major LPO product 4 HNE with nucleobases in hepatocytes of patients with ALD (28). In this context CYP2E1, induced by chronic alcohol consumption, significantly correlated with 4 HNE and εdA in liver biopsies from ALD patients, implying that CYP2E1 is an important factor in ethanol-mediated carcinogenesis (32). CYP2E1 is also induced in NAFLD (33-35). Although this induction is less pronounced as in ALD, it also has severe consequences with respect to the generation of oxidative stress. Indeed inflammation driven oxidative stress maybe an important mechanism in the progression of NAFLD.
Our data show clearly that εdA correlated significantly with the lipid peroxidation product 4 HNE, but not with CYP2E1. An explanation for that could be that the inflammatory process in our NAFLD patients resulting in ROS via cytokine driven inflammation predominates ROS generation by CYP2E1. Similar observations were made in children with NASH, where, in hepatic biopsies from NASH children only a borderline significance of εdA with CYP2E1 was found (36). It was speculated that most of ROS formation was rather inflammation driven than generated through CYP2E1, since it has been reported that high prevalence of oxidative stress in children with NAFLD is associated with an increased severity of steatohepatitis.
In an epidemiological retrospective study it was clearly shown that NASH patients had an increased risk to develop heptocellular cancer (37). And this was further enhanced, when alcohol was taken even in social quantities. In both situations, in NASH and following alcohol consumption, exocyclic etheno-DNA adducts occur and regardless of the course of formation of exocyclic etheno-DNA adducts, it is clear that these adducts are highly carcinogenic and are associated with an increased risk for hepatocarcinogenesis (37). Therefore, it was recommended patients with NAFLD should avoid alcohol of any amount (38).
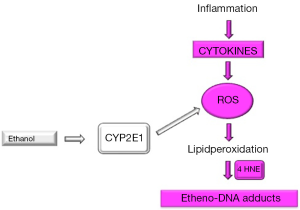
In summary, this is the first description of highly carcinogenic etheno-DNA adducts in NAFLD patients. We could show that εdA significantly correlated with lipid peroxidation product 4 HNE, but not with CYP2E1, implying that in NAFLD ROS generation with consecutive DNA damage is rather inflammation driven through various cytokines than by induction of CYP2E1. This context is illustrated in Figure 2.
It needs to be determined, whether these adduct early in the course of the disease may act as biological markers to predict a risk for development of hepatocellular cancer later in life.
Acknowledgements
Funding: This work was supported by the Dietmar Hopp Foundation.
Disclosure: The authors declare no conflict of interest.
Ethical Statement: The study was approved in accordance with the declaration of Helsinki by the Ethical Committee of the University of Heidelberg, Germany.
References
- Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis 1986;8:283-98. [PubMed]
- Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722-8. [PubMed]
- Suzuki A, Angulo P, Lymp J, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology 2005;41:64-71. [PubMed]
- Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology 2008;134:1682-98. [PubMed]
- Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 2007;11:1-16. [PubMed]
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917-23. [PubMed]
- Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434-8. [PubMed]
- Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology 2002;35:367-72. [PubMed]
- Cortez-Pinto H, Camilo ME, Baptista A, et al. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr 1999;18:353-8. [PubMed]
- Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002;35:373-9. [PubMed]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276-85. [PubMed]
- Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006;391:499-510. [PubMed]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749-59. [PubMed]
- Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 2006;65:278-90. [PubMed]
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 2007;7:599-612. [PubMed]
- Hiraku Y, Kawanishi S. Role of nitrative DNA damage in inflammation related carcinogenesis. In: Hiraku Y, Kawanishi S, Ohshima H. eds. Cancer and Inflammation Mechanisms: Chemical, Biological, and Clinical Aspects. Hoboken, NJ: John Wiley & Sons, 2014:41-59.
- Barbin A. Etheno-adduct-forming chemicals: from mutagenicity testing to tumor mutation spectra. Mutat Res 2000;462:55-69. [PubMed]
- Bartsch H, Barbin A, Marion MJ, et al. Formation, detection, and role in carcinogenesis of ethenobases in DNA. Drug Metab Rev 1994;26:349-71. [PubMed]
- Basu AK, Wood ML, Niedernhofer LJ, et al. Mutagenic and genotoxic effects of three vinyl chloride-induced DNA lesions: 1,N6-ethenoadenine, 3,N4-ethenocytosine, and 4-amino-5-(imidazol-2-yl)imidazole. Biochemistry 1993;32:12793-801. [PubMed]
- Pandya GA, Moriya M. 1,N6-ethenodeoxyadenosine, a DNA adduct highly mutagenic in mammalian cells. Biochemistry 1996;35:11487-92. [PubMed]
- Levine RL, Yang IY, Hossain M, et al. Mutagenesis induced by a single 1,N6-ethenodeoxyadenosine adduct in human cells. Cancer Res 2000;60:4098-104. [PubMed]
- Swenberg JA, Fedtke N, Ciroussel F, et al. Etheno adducts formed in DNA of vinyl chloride-exposed rats are highly persistent in liver. Carcinogenesis 1992;13:727-9. [PubMed]
- Lieber CS, DeCarli LM. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem 1970;245:2505-12. [PubMed]
- Cheng KC, Preston BD, Cahill DS, et al. The vinyl chloride DNA derivative N2,3-ethenoguanine produces G----A transitions in Escherichia coli. Proc Natl Acad Sci U S A 1991;88:9974-8. [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [PubMed]
- Eberle G, Barbin A, Laib RJ, et al. 1,N6-etheno-2'-deoxyadenosine and 3,N4-etheno-2'-deoxycytidine detected by monoclonal antibodies in lung and liver DNA of rats exposed to vinyl chloride. Carcinogenesis 1989;10:209-12. [PubMed]
- Yang Y, Nair J, Barbin A, et al. Immunohistochemical detection of 1,N(6)-ethenodeoxyadenosine, a promutagenic DNA adduct, in liver of rats exposed to vinyl chloride or an iron overload. Carcinogenesis 2000;21:777-81. [PubMed]
- Frank A, Seitz HK, Bartsch H, et al. Immunohistochemical detection of 1,N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis 2004;25:1027-31. [PubMed]
- Nair J, Nair UJ, Sun X, et al. Quantifying etheno-DNA adducts in human tissues, white blood cells, and urine by ultrasensitive (32)P-postlabeling and immunohistochemistry. Methods Mol Biol 2011;682:189-205. [PubMed]
- Tsutsumi M, Lasker JM, Shimizu M, et al. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology 1989;10:437-46. [PubMed]
- Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774-88. [PubMed]
- Wang Y, Millonig G, Nair J, et al. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 2009;50:453-61. [PubMed]
- Weltman MD, Farrell GC, Hall P, et al. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998;27:128-33. [PubMed]
- Abdelmegeed MA, Banerjee A, Yoo SH, et al. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol 2012;57:860-6. [PubMed]
- Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003;37:544-50. [PubMed]
- Qin H, Teufel U, Engelmann G, et al. Detection of hepatuic highly carcinogenic, exocyclic etheno DNA adducts in patients with alcoholic and non-alcoholic fatty liver disease and in children with non-alcoholic steatohepatitis. J Hepatology 2012;56:S520.
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [PubMed]
- Liangpunsakul S, Chalasani N. What should we recommend to our patients with NAFLD regarding alcohol use? Am J Gastroenterol 2012;107:976-8. [PubMed]


