STRA6: role in cellular retinol uptake and efflux
Introduction
Vitamin A (all-trans-retinol) is an essential nutrient present in our diet in the form of carotenoids (provitamin A) in plant products and as retinyl esters (RE) (preformed vitamin A) in animal products (1). This isoprenoid lipid plays critical roles throughout the vertebrate life cycle in physiological processes as diverse as embryonic development, vision, and cell homeostasis (2-4). Vitamin A is the precursor for at least two critical metabolites, all-trans-retinoic acid (RA) and 11-cis-retinal (5-7) (Figure 1). RA is a hormone-like compound that binds to transcription factors referred to as retinoic acid receptors (RARs) in many cell types and tissues. RARs in conjunction with its heterodimeric partner retinoid X receptors (RXRs) control the activity of diverse target genes throughout the mammalian life cycle (8). 11-cis-Retinal is the chromophore of visual pigments in photoreceptor cells of the retina (9). These G protein coupled receptors mediate phototransduction by the process in which light is translated into a nervous stimulus (10).
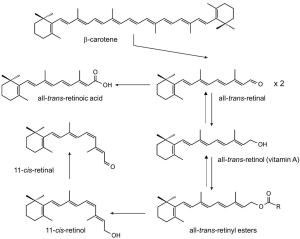
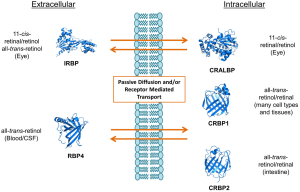
To become biologically active, vitamin A and its dietary precursors must be absorbed in the intestine, metabolically transformed and conveyed to the eyes and other tissues. But the hydrophobicity of carotenoids and retinoids presents a physical barrier for simple aqueous diffusion. Thus animals have evolved specific proteins that bind and solubilize these lipids in extra- and intracellular compartments of the body. In humans and other mammals, intracellular and extracellular proteins exist that specifically bind to various vitamin A derivatives, including all-trans-retinol and retinal, 11-cis-retinol and retinal, and all-trans-RA (Figure 2). Retinoid binding proteins including retinol binding protein (RBP), which binds to retinol in the circulation, protect these lipids and their surroundings from chemical modification due to their chemical reactivity. Though some researchers propose that retinoids can exchange between RBP and membranes (11,12), increasing evidence indicates that membrane transport and cellular uptake of carotenoids and retinoids is facilitated by transporters such as the scavenger receptor class B type 1 (13), the ATP-binding cassette transporter ABCA4 (14) and the channel-like membrane protein encoded by the stimulated by retinoic acid 6 (STRA6) gene product (15). In recent years, these components have attracted broad scientific and clinical interest since they are associated with disease, including Stargardt disease (16,17), diabetes mellitus (18) and complex microphthalmic syndromes (19,20). This review will discuss recent research related to the biochemistry and physiology of vitamin A transport with a particular focus on the multidomain membrane protein STRA6.
The discovery of an RBP receptor
For years, scientists wondered how retinol bound to RBP in the blood was able to cross cell membranes to enter the cytoplasm of target cells. In the mid 1970’s, two groups independently reported evidence for the existence of a cellular receptor for RBP. This molecule was present on the basolateral surface of bovine retinal pigment epithelium (RPE) cells (21,22) as well as on cells of the monkey’s small intestine (23). The interaction between RBP and this receptor is highly specific, and was shown to behave in a saturable, temperature dependent manner (21,23). This cell surface RBP receptor not only specifically binds to RBP but also mediates the uptake of the cargo from vitamin A—loaded RBP (holo-RBP) into cells (24). Since then receptor mediated vitamin A uptake has been demonstrated for other tissues and cell types including the Sertoli cells of the testis, the placenta, the choroid plexus of the brain, and human skin (24-29). Based on these observations the RBP receptor may play a particular role for vitamin A transport in epithelia that that constitute blood/tissue barriers.
It wasn’t until over 30 years after the first biochemical description of the RBP receptor that the molecular nature of this protein was identified as STRA6. This breakthrough was achieved using an elegant strategy that stabilized the fragile interaction between recombinant tagged RBP and STRA6 by protein cross-linking followed by high affinity purification of the complex (30). Cell culture studies revealed that STRA6 fulfills all three criteria expected for a bona fide RBP membrane receptor: (I) RBP binds to this membrane protein; (II) STRA6 mediates cellular uptake of vitamin A; and (III) STRA6 is expressed in many tissues in which the native receptor has been previously described including RPE, the choroid plexus of the brain, and the Sertoli cells of the testis (30). It was also shown that STRA6 is expressed in other tissues such as the hippocampus and spleen (30).
STRA6 was identified in 1995 as a gene with unknown function which is transcriptionally up-regulated by RA exposure (31). Expression patterns were further characterized two years later in murine embryos and adults, where STRA6 was shown to be expressed in heart, lung, female genital tract, kidneys, and spleen, as well as during embryonic development (32). In embryonic F9 cells, STRA6 gene expression is preferentially mediated by the RARγ/RXRα heterodimer, and STRA6 expression was shown to be repressed in the presence of RARα (31).
STRA6 homologues are encoded in all known genomes of vertebrates (33). Recently, it has been suggested that in some vertebrates, the STRA6 gene has undergone positive selection during evolution. This selection was proposed to ultimately affect visual acuity of the species (33) due to STRA6’s pivotal role in ocular vitamin A uptake. However, there is no experimental evidence to support this notion and thus further biochemical studies are necessary.
STRA6 biochemistry
After the molecular identification of STRA6 as an RBP receptor, several attempts have been made to determining the mechanism by which vitamin A bound to RBP is released and taken up by cells. Determining biochemical properties of STRA6 has been a fast paced, competitive research area in recent years. The following summary gives a short overview of this research field, though many questions still remain unanswered.
To understand the mechanism of STRA6-mediated vitamin A uptake, information regarding the structure of this membrane protein is required. So far, the field lacks a high resolution structure of STRA6, and structural predictions are hampered by the absence of known STRA6-related proteins in nature. Computer simulations suggest that STRA6 contains eleven transmembrane segments, however evidence from domain-tagging experiments indicate that it contains only nine segments (34,35). A short N-terminal tail faces the extracellular space, while a long, possibly unstructured C-terminal tail faces the cytosol. Two large intracellular loops and one large extracellular loop are also present (36), along with several smaller loops alternating to face each side of the membrane (34,35). The nine transmembrane segment model of STRA6 is shown in Figure 3, though the eleven transmembrane model is also used for structural prediction by some researchers (37,38).
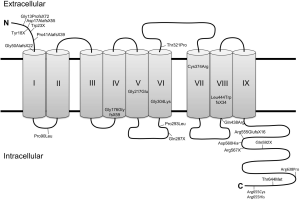
The extracellular region of STRA6 binds quite tightly to RBP. When the first evidence for a cell surface receptor for RBP was provided, the dissociation constant of the native receptor was determined to be 5 pM (21). Later, the KD for recombinant STRA6 was determined to be somewhat weaker, albeit still quite strong at 59 nM (30). Site directed mutagenesis of recombinant STRA6 indicate that RBP binds to the large extracellular loop between transmembrane segments 6 and 7. Three essential residues (Tyr336, Gly340, and Gly342) are absolutely necessary for this interaction, and mutation in any of these residues results in an inability for RBP to bind with STRA6 (34).
After exploring structural features of STRA6, we may now discuss the mechanism of transport. Inhibitors of endocytosis do not affect STRA6 activity. This suggests that the uptake of RBP-bound retinol via STRA6 does not depend on endocytosis of the complex (30). Also, since retinol is not present as a free molecule in the blood and has no charge, this transport is not fueled by an electrochemical gradient (39). There must be some other mechanism by which STRA6 shuttles vitamin A into the cell. Several studies showed that STRA6 functions efficiently only when coupled to other proteins which facilitate vitamin A storage within the cell. Lechithin retinol acyl transferase (LRAT) stimulates STRA6 activity, and is important for the transport of retinol across the cell membrane (30,40). Cellular retinol binding protein-1 (CRBP1), a protein which binds to and solubilizes retinol inside the cell, is another protein associated with vitamin A storage which affects the function of STRA6 (39) (Figure 4). STRA6 loads retinol to CRBP1 but not CRBP2, suggesting that there must be some factor other than retinol binding ability that is required to enhance STRA6 activity (39,41). In fact when LRAT and CRBP1 are not present, retinol uptake by STRA6 is very low (39,40). Though a binding site for CRBP1 has not been identified on the STRA6 protein (42,43), it would likely be located at the C-terminal region of STRA6. The idea that the C-terminus plays a particular role for STRA6 functioning is corroborated by the observation that mutations in this region can cause the debilitating and sometimes fatal Mathew Wood Syndrome (MWS) in humans (see below). Elucidation of the structural organization of intracellular C-terminal domain of STRA6 would provide valuable insight regarding the mechanism of STRA6-mediated vitamin A transport and its interaction with intracellular vitamin A processing proteins.
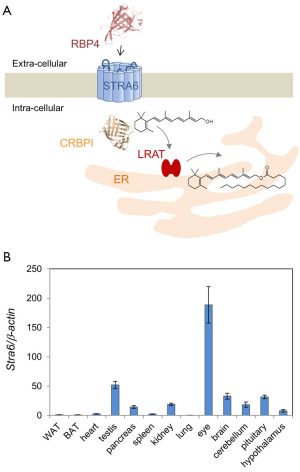
In cells expressing LRAT, retinol is converted to RE, seemingly preventing the saturation of the uptake process (40). When the ratio of holo-RBP to STRA6 is larger, the function of STRA6 becomes more dependent on LRAT, possibly because all-trans-retinol in the cell inhibits further retinol intake (39). This functional coupling of STRA6 and LRAT in vitamin A uptake homeostasis has also been demonstrated in mice. Mice lacking LRAT have significantly impaired retinol uptake (44). Moreover, it has been shown that this coupling is subject to regulation by RA (44).
STRA6 works in reverse as well. In the presence of apo-RBP, STRA6 promotes the loading of retinol onto RBP, a process which is inhibited by the presence of LRAT (39,40). Thus, it was suggested that STRA6 operates as a pore through which retinol can travel, and the path of retinol extends as far as the intracellular loops of STRA6 (45). Interestingly, STRA6 activity can be inhibited by free retinol outside the cell. However, the presence of LRAT and CRBP1 reduces this inhibition, as the retinol inside the cell is then converted to RE, keeping intracellular binding sites available for retinol entering via STRA6 (39).
In the circulation, holo-RBP is bound to transthyretin (TTR), also known as prealbumin (46). The formation of this ternary complex is required to prevent glomerular filtration of the relatively small holo-RBP molecule (47,48). The binding of TTR to apo-RBP is not as strong as that of TTR to holo-RBP, which is consistent with the idea that this association ensures the precious retinol is not lost as it passes through the kidney (49). As with holo-RBP not bound to TTR, STRA6 mediates transport of retinol from the holo RBP-TTR complex (39). It has been suggested that TTR can partially suppress STRA6 activity, although retinol is still transported in this case (30,37). Experimental evidence from cell culture further suggests that TTR inhibits the import of retinol via STRA6 in the absence of LRAT or CRBP1 (39).
STRA6 preferentially mediates the uptake of all-trans-retinol bound to RBP. However, it also transports RBP-bound long chain apocarotenoids such as 10-apocarotenol though less efficiently when compared to retinol (50). In contrast, RBP-bound all-trans-RA is not a substrate for STRA6 (40). Several factors have been described that can affect cellular retinol uptake. For instance, it has been shown for STRA6-mediated release of retinol from holo-RBP, as well as STRA6-mediated loading of retinol into apo-RBP, there are isomer specific effects. In cell culture experiments, the addition of fenretinide, all-trans-retinal, all-trans-RA, or 13-cis-RA promoted the release of retinol mediated by STRA6. Fenretinide, all-trans-retinal, 9-cis-retinal, all-trans-RA and 13-cis-RA have been shown to inhibit the STRA6-catalyzed loading of retinol onto apo-RBP in the same study. Finally, that study showed that fenretinide, 9-cis-retinal and all-trans-RA promote STRA6-mediated transport of retinol from holo-RBP to CRBP1, while all-trans-retinal inhibits this activity (51). It has also been shown that β-ionone, a molecule found in produce with a structure similar to that of retinoids, promotes uptake of retinol via STRA6 while at the same time inhibiting the loading of retinol onto RBP mediated by STRA6 (39). This is interesting, because β-ionone can be present in foods rich in vitamin A in the form of carotenoids and is endogenously produced by the carotene-9,10-oxygenase (52). Moreover, some researchers suggest that the large C-terminal intracellular loop of STRA6 may not only play a role for accommodating retinol and delivering it to CRBP1 but may also interact with other cellular components (36). Further studies are needed to unveil the biochemistry of retinol transport and vitamin A uptake homeostasis in molecular and structural detail.
Another way in: RBPR2
Previously, the existence of a second RBP receptor expressed in tissues lacking STRA6 was postulated (53), and in 2013, RBPR2 was molecularly identified (14,54). RBPR2 is a single chain protein in mice, but in humans and other primates is a double chain protein where the chains are encoded by two separate genes in different loci (54). The primary amino acid sequence of RBPR2 has less than 18% overall homology to STRA6, with some short segments displaying over 50% amino acid sequence homology. Computer simulations predict that RBPR2 has a structure related to that of STRA6, with 9 to 11 predicted transmembrane segments. It also shares conserved amino acids with STRA6 which are essential for either the structure or function of these proteins. Cell culture studies showed that RBPR2 is localized to the membrane, displays high affinity binding to RBP, and transports retinol from holo-RBP into cells (54). Thus, RBPR2 fulfills the key biochemical criteria of an RBP receptor described above. RBPR2 mRNA is expressed in the liver, small intestine, jejunum and ileum, and to a lesser degree in the spleen and colon of wild type mice. It is also expressed in adipocytes, and this expression in fat is induced in obese mice (54). The existence of this protein is a likely explanation for why the liver and other tissues can readily uptake vitamin A from holo-RBP despite lacking STRA6 expression.
STRA6 and vitamin A homeostasis: studies in animal models
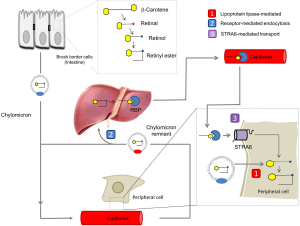
Dietary vitamin A is absorbed in the intestine, esterified and transported along with other dietary lipids in triacylglycerol-rich chylomicrons (55,56). Some vitamin A from circulating chylomicrons is taken up by peripheral tissues in a lipoprotein lipase-dependent manner (57,58). The majority is taken up by the liver for storage as RE in Stellate cells (55). Stored vitamin is released from hepatocytes in a RBP-dependent manner and holo-RBP is the major transport mode of vitamin A in the fasting circulation (57). Thus we are left with a critical question: why have vertebrates developed two different transport systems for vitamin A (for the current model see Figure 5)? An obvious explanation is that the RBP-dependent system helps vertebrates to endure periods of dietary vitamin A deprivation by distributing stored vitamin A. Although this argument certainly is valid, the RBP-dependent transport system offers additional advantages such as tissue specificity and regulation of vitamin A supply of the periphery. The identification of STRA6 as an RBP receptor paved the way to analyze the molecular underpinning of this process. The major findings in two different animal models, zebrafish and mice, are briefly discussed below.
The zebrafish model
In recent years, zebrafish (Danio rerio) has become a key model to study vertebrate development (59). The fish develops within five days from a fertilized oocyte to a free-swimming larva, with notably well-developed eyes and sight (60). To support this process, the yolk contains a considerable amount of maternal vitamin A that must be distributed within the developing embryo for the production of vitamin A hormone in most embryonic compartments and visual chromophore in developing photoreceptor cells (61,62). A need to coordinate this entire process becomes evident by the different quantitative requirements for the vitamin for gene regulation and chromophore production. Herein, the large majority of yolk vitamin A (more than 90%) is transported to the developing eyes (61). Loss-of-function studies revealed that RBP and STRA6 play a pivotal role in this transport process (40). Zebrafish STRA6 and RBP share overall amino acid sequence homology with the mammalian proteins, including the trans-membrane segments and C-terminal domain. Stra6 is expressed relatively late during fish development, most pronounced in developing light sensitive organs such as the pineal gland and the RPE of the eyes (40). Previous studies revealed that these light sensitive organs also express other key components for vitamin A uptake and visual chromophore production such as LRAT and the retinoid isomerase RPE65 (63,64). The fish produces RBP in cells of the yolk syncytial layer starting with 24 hours past fertilization, indicating that this carrier protein helps to mobilize yolk vitamin A for transport in the developing circulation (40). Indeed, gene knockdown of STRA6 provided in vivo evidence for this assumption and demonstrated that the eyes of STRA6-deficient larvae suffered from vitamin A deficiency. These larvae also displayed characteristic embryonic malformations such as microphthalmia and a curved body axis (40). Additional malformations were observable in the developing cardiovascular system such edema of the pericardium, dysmorphic heart chambers, and peripheral bleedings. Although the hearts of STRA6-deficient fish larvae were still able to contract, most fish did not display a circulating blood stream (40). Interestingly, marker gene analysis revealed that disruption of STRA6 resulted in a complex vitamin A phenotype. STRA6 expressing tissues such as the eyes suffered from vitamin A-deficiency, whereas other embryonic compartments, e.g., the region where the heart develops, suffered from vitamin A excess (40). Notably, the blocking of RBP-dependent embryonic vitamin A transport either by 1-phenyl-2-thiourea treatment or by antisense oligonucleotides directed against RBP significantly alleviated malformations in the majority of STRA6-deficient fish larvae (40). Thus, studies in zebrafish indicate that STRA6 plays a vital role for vitamin A homeostasis during the development of oviparous vertebrates. In the developing eyes, the RBP receptor is required for vitamin A uptake from circulating holo-RBP. Its absence causes retinoid-deficiency and characteristic ocular malformations of the retina. Other tissues, however, suffer from excess RA production since yolk vitamin A is no longer delivered to its ocular target tissue and biochemically converted to chromophore.
Mouse models
Mammals depend on a constant vitamin A supply to support retinoid signaling during embryonic and fetal development. However, the vitamin A demand of the developing visual system differs significantly between mammalian species. For instance, rodents are born blind without differentiated photoreceptors, whereas primates including monkeys already have sight at birth. To analyze the role of STRA6 for mammalian vitamin A homeostasis, three laboratories have independently established STRA6-/- mice by conventional gene knockout approaches (65-67). Additionally, a conditional knockout mouse model for STRA6 has been established and the role of STRA6 in adipocyte biology has been studied (68). STRA6 null mice are viable and born at Mendelian frequency (65-67). To the naked eye, STRA6 knockout mice appear similar to normal mice. Consistent with the expression in the developing eyes, STRA6-deficient animals display ocular malformations that are characteristic for vitamin A deficient animals. These mice show a slight reduction in eye diameter (65,69) and have shortened inner and outer segments of photoreceptors (65,67). In one study, the eyes of the knockout mice were reported to display a persistent hyperplastic primary vitreous (65,69). In another study, choroidal coloboma have been reported in a STRA6-defiecint mouse line (67). It remains to be clarified whether these pathologies are manifestation of the same developmental defect. In ERG analyses, knockout mice have largely reduced a and b waves, indicating a significant impairment in phototransduction of cone and rod photoreceptors (65,67). Consistently STRA6-deficient mice display largely reduced levels of all ocular retinoid cycle intermediates when compared to heterozygous siblings. Blood and other tissues such as the lungs, fat and liver display normal vitamin A levels when mice are bred and raised on vitamin A sufficient diets (65,67,69). A notable exception seems to be the brain. As indicated by an accumulation of RBP in the ependymal cells of the choroid plexus, vitamin A uptake homeostasis of this organ is seemingly impaired (67). Notably, treatment with pharmacological doses of vitamin A triggered RBP release from these cells into the cerebrospinal fluid (67). Furthermore, such treatment increased ocular vitamin A levels and restored ERG responses in STRA6-deficient animals, thus demonstrating that vitamin A can diffuse through the blood-retina and blood-retinal barriers when present in pharmacological amounts (67).
STRA6-/- mice were also employed to test the role of this membrane protein in the adult. Thus, mice were subjected either to dietary vitamin A restriction or raised on diets copious for the vitamin. Ocular retinoid content of STRA6-/- mice increased during adolescence when mice were kept on a high vitamin A diet (67), a phenomenon previously described in RBP-deficient mice (57). Most other peripheral tissues did not display altered retinoid levels in STRA6-deficiency when compared to heterozygous siblings (67). A minor role of STRA6 in vitamin A uptake is consistent with the very low expression levels of STRA6 mRNA in most peripheral tissues (67). These observations clearly indicate that vitamin A can be delivered to these peripheral tissues by mechanisms that do not involve the retinoid transport channel STRA6, and this process likely depends on dietary vitamin A from chylomicrons (57,67). This picture changes when mice are subjected to dietary vitamin A restriction during adolescence. In STRA6-/- mice, ocular vitamin A content continuously decreased under this restriction and was below detection level in some animals after 14 weeks of deprivation. In contrast, ocular vitamin A content even increased in mice carrying a wild type STRA6 allele (67). This finding indicates that STRA6 is mandatory for ocular retinoid vitamin A uptake from circulating RBP that constitutes the major transport mode for vitamin A during dietary deficiency. The increase in ocular vitamin A in mice carrying a wild type STRA6 allele under dietary vitamin A deprivation was mirrored by a decrease in hepatic and pulmonary retinoid levels, indicative of a re-distribution of the vitamin from its storage compartments to the eyes (44,67).
The preferential delivery of vitamin A to the eyes under dietary restriction might be explained by a diet responsive regulation of this process. In peripheral tissues such as the lung, both STRA6 and LRAT are RA-inducible genes (44,70,71). Thus treatment with compounds that induces the expression of these genes result in a rapid clearance of vitamin A from holo-RBP from the circulation and the storage of vitamin A in the form of RE (44,67). In the eyes, STRA6 and LRAT mRNA expression occurs largely independent of RA and this process is not influenced by the vitamin A status of the diet (44).
Though significant progress has been made in elucidating the path for vitamin A delivery to the periphery, several questions about STRA6 biology remain to be answered. For instance, it needs to be clarified whether STRA6 is mandatory for vitamin A uptake homeostasis of other epithelia that constitute a blood-tissue barrier such as the ependymal cells of the choroid plexus and the Sertoli cells of the testis. Additionally, the role of STRA6 in organs with relatively high mRNA expression such as the brain, pancreas, and spleen needs to be further defined. Moreover, STRA6’s contribution to vitamin A homeostasis of epidermal and mesenchymal cell layers of the skin deserves further attention (72). Finally, it remains to be addresses whether STRA6-mediated transport works in both directions in the in vivo situation, thereby mediating a constant exchange of vitamin A between tissues and circulating RBP. Such leveling of retinoid tissue levels by STRA6 was recently indicated in adipocyte differentiation (73).
STRA6 and disease
Largely independent of research about the biochemistry of vitamin A transport, the clinical relevance of this process has been discovered. Diseases associated with mutations in the STRA6 gene are rare but severe and their phenotypic manifestations are consistent with the essential role of vitamin A and RBP in vertebrate development (54-58). Moreover, blood vitamin A homeostasis has been shown to be significantly altered in patients that suffer from type 2 diabetes. Under this condition the levels of RBP are significantly increased and some studies suggest a causative relation between RBP and insulin resistance. Thus, molecular factors affecting this process have attracted broad clinical interest.
Mutations in STRA6 cause the fatal MWS
In 1933, Blackfan et al. (74) summarized early observations suggesting vitamin A to be an essential part of the diet for maintaining good health. They noted that children who did not ingest adequate amounts of vitamin A displayed high incidences of infections, opthalmia, an emaciated appearance and night blindness. These cases were observed to be extremely rare prior to weaning off breast milk. Retrospectively, the observed variability in clinical manifestations is indicative of the complex biochemical interactions surrounding retinoid metabolism being studied today.
Also known as human microphthalmic syndrome 9 (MCOPS9), Spears syndrome, and PDAC, MWS is characterized by severe bilateral microphthalmia, often in combination with pulmonary dysplasia, cardiac defects, and diaphragmatic hernia. Additionally, brain anomalies, mental retardation and malformations of the kidneys, pancreas and gastrointestinal tract have been described in affected individuals (75-77). In 2007, two groups reported independently that STRA6 mutations are the cause of this complex inherited disease (19,20). One year later, several more STRA6 sequence variants were identified in patients displaying either anophthalmia or microphthalmia, concluding that by changing the structure of the STRA6 protein, these variants could influence the pathogenesis of these malformations (78). Because nonsense mutations throughout the entire STRA6 gene can cause MWS, the STRA6 protein must be likely full length in order to be functional. Additionally, there are disease-causing mutations along the unusually long C-terminus tail of the STRA6 protein, thus implicating an important functional role of this intracellular domain. Cell culture studies with recombinant STRA6 demonstrated that disease-causing missense mutations either interfered with intracellular protein transport or significantly reduced vitamin A uptake activity (34).
Today 24 missense and nonsense mutations in STRA6 have been identified that are associated with the clinical symptoms of MWS and early childhood death (Figure 3). However, survivors with STRA6 null mutations also have been identified in genetic screens of patients with isolated microphthalmia (79). Variability in the clinical manifestations of STRA6 mutations has even been found in consanguine family members. In an Irish family, the same homozygous missense mutation was manifested as either fatal MWS or an isolated microphthalmia (80). Thus, a clear genotype/phenotype association has not been established for STRA6 mutations.
Surprisingly, compound missense mutations in the human RBP gene manifest as much milder ocular defects and night blindness (81,82). In several respects, this phenotype resembles the phenotypes of STRA6 and RBP null mice (67). The milder phenotype of the RBP mutations might indicate that the mutant RBP still transports enough vitamin A to support vitamin A-dependent eye development and differentiation. The more severe ocular malformations in STRA6-deficient patient suggest differences in vitamin A biology. This assumption is corroborated by recent genetic evidence showing that mutations in the gene encoding the retinoic acid producing enzyme ALDH1a3 cause a similar eye phenotype in humans (83), whereas ALDH1a3 knockout mice display no pronounced microphthalmia (84,85). Additionally, the extra-uterine eye development and delivery of dietary vitamin A via breast milk may make the developing murine eyes less dependent on STRA6.
The discrepancy between the clinical manifestations of mutations in RBP and STRA6 in humans has led to the speculation that additional genetic alterations must be present in MWS patients (69). However, this variability may also reflect maternal vitamin A status and delivery to the fetus. Studies in mice indicate that both dietary and stored vitamin A can be transported through the fetal-maternal-blood barrier (86,87). Notably, dietary vitamin A restriction of RBP knockout mice leads to malformations in the offspring that resemble the birth defects described in MWS patients (86). Biochemical evidence has been provided that an RBP receptor is expressed in the placenta that mediates vitamin A transport (41). In mice it is yet not clear whether this receptor is STRA6 or the recently identified RBPR2, since they are both expressed in this tissue (30,54,88). Variation of dietary vitamin A supply during critical periods of pregnancy could be an important modulator of MWS severity. Thus treatment with pharmacological doses of vitamin A might alleviate the disease phenotype.
STRA6 and diabetes
Large epidemiologic studies suggest that elevated blood RBP levels are associated with insulin resistance, prediabetes, and the metabolic syndrome (18). Genetic studies associate polymorphism in the RBP gene with increased risk of type 2 diabetes (89-91). However, the mechanism by which RBP induces insulin resistance in peripheral tissues is a matter of debate. Some studies suggest a possible role for pro-inflammatory pathways in this process. RBP levels in serum and adipose tissue strongly correlate with subclinical inflammation, including pro-inflammatory cytokine levels. Cell culture studies indicate that RBP can indirectly impair insulin signaling in adipocytes by inducing pro-inflammatory cytokine production from macrophages (92). Accordingly, ectopic expression of RBP in mice results in adipocyte inflammation by activating innate immunity that elicits an adaptive immune response, eventually causing insulin resistance of adipose tissue (93). While these effects of RBP are vitamin A-independent, vitamin A-dependent mechanisms have also been proposed. Some researchers suggested that the holo-RBP complex mediates insulin resistance in adipocytes by stimulating JAK2/STAT5 signaling via STRA6 and expression of suppressor of cytokine signaling 3 (36). Thus it was proposed that STRA6 is a cytokine receptor that couples vitamin A transport with cellular signaling rather than acting as a bona fide transporter (37,38,69). However, this claim is controversial because of several experimental inconsistencies in these studies, including the use of a nonspecific antibody for the detection of human STRA6 (42,43). Though the authors acknowledged the dependency of STRA6 on CRBP1 and LRAT, this proposal contradicts the established biochemical properties of STRA6, including its ability to act as a bidirectional retinoid channel (42,43). Additionally, a role of STRA6 as a mediator of insulin resistance is not supported by what is known about STRA6 in adipocyte biology. In adipocytes, STRA6 expression is very low and further declines in obesity, thus excluding a participation in pathologies associated with this condition (68). Furthermore, STRA6 expression is induced by RA in fat (44) and RA rather improves than deteriorates insulin sensitivity in rodents (94). The latter observation lends itself to another path explaining how STRA6 might be connected to insulin resistance. Increased RBP blood levels in diabetic subjects may reflect impaired vitamin A uptake and metabolism in peripheral tissues such as fat. Several studies demonstrated that vitamin A plays an important role for adipocyte differentiation and functioning (94-97). In fact, natural and synthetic vitamin A derivatives such as RA and fenretinide induce STRA6 expression in the periphery, promote vitamin A uptake, lower blood RBP, and improve insulin sensitivity in mice (44,98). Further studies are required to clarify the role of STRA6 in disease states associated with increased blood RBP levels.
Acknowledgements
Funding: Studies about STRA6 biochemistry and function conducted by the von Lintig laboratory were supported by U.S. National Institute of Health grants (EY019641, EY020551). Mary Kelly is supported by the Visual Sciences Training Program grant from the National Institutes of Health (T32 EY007157).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 2010;30:35-56. [PubMed]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008;134:921-31. [PubMed]
- Rhinn M, Dollé P. Retinoic acid signalling during development. Development 2012;139:843-58. [PubMed]
- von Lintig J. Metabolism of carotenoids and retinoids related to vision. J Biol Chem 2012;287:1627-34. [PubMed]
- Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem Rev 2014;114:194-232. [PubMed]
- Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta 2012;1821:152-67.
- Kedishvili NY. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res 2013;54:1744-60. [PubMed]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 2006;46:451-80. [PubMed]
- Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem 2006;75:743-67. [PubMed]
- Arshavsky VY, Lamb TD, Pugh EN Jr. G proteins and phototransduction. Annu Rev Physiol 2002;64:153-87. [PubMed]
- Noy N, Blaner WS. Interactions of retinol with binding proteins: studies with rat cellular retinol-binding protein and with rat retinol-binding protein. Biochemistry 1991;30:6380-6. [PubMed]
- Noy N, Slosberg E, Scarlata S. Interactions of retinol with binding proteins: studies with retinol-binding protein and with transthyretin. Biochemistry 1992;31:11118-24. [PubMed]
- Reboul E, Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res 2011;50:388-402. [PubMed]
- Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res 2010;49:476-92. [PubMed]
- Sun H, Kawaguchi R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int Rev Cell Mol Biol 2011;288:1-41. [PubMed]
- Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 1997;277:1805-7. [PubMed]
- Allikmets R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997;17:122. [PubMed]
- Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552-63. [PubMed]
- Pasutto F, Sticht H, Hammersen G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 2007;80:550-60. [PubMed]
- Golzio C, Martinovic-Bouriel J, Thomas S, et al. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet 2007;80:1179-87. [PubMed]
- Heller J. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J Biol Chem 1975;250:3613-9. [PubMed]
- Heller M, Bok D. A specific receptor for retinol binding protein as detected by the binding of human and bovine retinol binding protein to pigment epithelial cells. Am J Ophthalmol 1976;81:93-7. [PubMed]
- Rask L, Peterson PA. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey's small intestine. J Biol Chem 1976;251:6360-6. [PubMed]
- Sivaprasadarao A, Findlay JB. The interaction of retinol-binding protein with its plasma-membrane receptor. Biochem J 1988;255:561-9. [PubMed]
- Sivaprasadarao A, Findlay JB. Structure-function studies on human retinol-binding protein using site-directed mutagenesis. Biochem J 1994;300:437-42. [PubMed]
- Bhat MK, Cama HR. Gonadal cell surface receptor for plasma retinol-binding protein. A method for its radioassay and studies on its level during spermatogenesis. Biochim Biophys Acta 1979;587:273-81. [PubMed]
- MacDonald PN, Bok D, Ong DE. Localization of cellular retinol-binding protein and retinol-binding protein in cells comprising the blood-brain barrier of rat and human. Proc Natl Acad Sci U S A 1990;87:4265-9. [PubMed]
- Båvik CO, Peterson A, Eriksson U. Retinol-binding protein mediates uptake of retinol to cultured human keratinocytes. Exp Cell Res 1995;216:358-62. [PubMed]
- Smeland S, Bjerknes T, Malaba L, et al. Tissue distribution of the receptor for plasma retinol-binding protein. Biochem J 1995;305:419-24. [PubMed]
- Kawaguchi R, Yu J, Honda J, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007;315:820-5. [PubMed]
- Bouillet P, Oulad-Abdelghani M, Vicaire S, et al. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev Biol 1995;170:420-33. [PubMed]
- Bouillet P, Sapin V, Chazaud C, et al. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 1997;63:173-86. [PubMed]
- Wu J, Xiang H, Qi Y, et al. Adaptive evolution of the STRA6 genes in mammalian. PLoS One 2014;9:e108388. [PubMed]
- Kawaguchi R, Yu J, Wiita P, et al. An essential ligand-binding domain in the membrane receptor for retinol-binding protein revealed by large-scale mutagenesis and a human polymorphism. J Biol Chem 2008;283:15160-8. [PubMed]
- Kawaguchi R, Yu J, Wiita P, et al. Mapping the membrane topology and extracellular ligand binding domains of the retinol binding protein receptor. Biochemistry 2008;47:5387-95. [PubMed]
- Berry DC, Jin H, Majumdar A, et al. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci U S A 2011;108:4340-5. [PubMed]
- Berry DC, Croniger CM, Ghyselinck NB, et al. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol-binding protein receptor STRA6. Mol Cell Biol 2012;32:3851-9. [PubMed]
- Berry DC, O'Byrne SM, Vreeland AC, et al. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol Cell Biol 2012;32:3164-75. [PubMed]
- Kawaguchi R, Yu J, Ter-Stepanian M, et al. Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem Biol 2011;6:1041-51. [PubMed]
- Isken A, Golczak M, Oberhauser V, et al. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab 2008;7:258-68. [PubMed]
- Redondo C, Vouropoulou M, Evans J, et al. Identification of the retinol-binding protein (RBP) interaction site and functional state of RBPs for the membrane receptor. FASEB J 2008;22:1043-54. [PubMed]
- Zhong M, Kawaguchi R, Kassai M, et al. Apo-RBP, holo-RBP, and insulin resistance. Mol Cell Biol 2014;34:2105-6. [PubMed]
- Zhong M, Kawaguchi R, Kassai M, et al. How free retinol behaves differently from rbp-bound retinol in RBP receptor-mediated vitamin A uptake. Mol Cell Biol 2014;34:2108-10. [PubMed]
- Amengual J, Golczak M, Palczewski K, et al. Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J Biol Chem 2012;287:24216-27. [PubMed]
- Zhong M, Kawaguchi R, Ter-Stepanian M, et al. Vitamin A transport and the transmembrane pore in the cell-surface receptor for plasma retinol binding protein. PLoS One 2013;8:e73838. [PubMed]
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest 1968;47:2025-44. [PubMed]
- Episkopou V, Maeda S, Nishiguchi S, et al. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci U S A 1993;90:2375-9. [PubMed]
- van Bennekum AM, Wei S, Gamble MV, et al. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem 2001;276:1107-13. [PubMed]
- Fex G, Albertsson PA, Hansson B. Interaction between prealbumin and retinol-binding protein studied by affinity chromatography, gel filtration and two-phase partition. Eur J Biochem 1979;99:353-60. [PubMed]
- Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, et al. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem 2013;288:34081-96. [PubMed]
- Kawaguchi R, Zhong M, Kassai M, et al. Differential and isomer-specific modulation of vitamin A transport and the catalytic activities of the RBP receptor by retinoids. J Membr Biol 2013;246:647-60. [PubMed]
- Kiefer C, Hessel S, Lampert JM, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110-6. [PubMed]
- Gjøen T, Bjerkelund T, Blomhoff HK, et al. Liver takes up retinol-binding protein from plasma. J Biol Chem 1987;262:10926-30. [PubMed]
- Alapatt P, Guo F, Komanetsky SM, et al. Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J Biol Chem 2013;288:1250-65. [PubMed]
- O'Byrne SM, Wongsiriroj N, Libien J, et al. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 2005;280:35647-57. [PubMed]
- Wongsiriroj N, Piantedosi R, Palczewski K, et al. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem 2008;283:13510-9. [PubMed]
- Quadro L, Blaner WS, Salchow DJ, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633-44. [PubMed]
- van Bennekum AM, Fisher EA, Blaner WS, et al. Hydrolysis of retinyl esters by pancreatic triglyceride lipase. Biochemistry 2000;39:4900-6. [PubMed]
- Ingham PW. Zebrafish genetics and its implications for understanding vertebrate development. Hum Mol Genet 1997;6:1755-60. [PubMed]
- Fleisch VC, Neuhauss SC. Parallel visual cycles in the zebrafish retina. Prog Retin Eye Res 2010;29:476-86. [PubMed]
- Lampert JM, Holzschuh J, Hessel S, et al. Provitamin A conversion to retinal via the beta,beta-carotene-15,15'-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 2003;130:2173-86. [PubMed]
- Costaridis P, Horton C, Zeitlinger J, et al. Endogenous retinoids in the zebrafish embryo and adult. Dev Dyn 1996;205:41-51. [PubMed]
- Isken A, Holzschuh J, Lampert JM, et al. Sequestration of retinyl esters is essential for retinoid signaling in the zebrafish embryo. J Biol Chem 2007;282:1144-51. [PubMed]
- Schonthaler HB, Lampert JM, Isken A, et al. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur J Neurosci 2007;26:1940-9. [PubMed]
- Ruiz A, Mark M, Jacobs H, et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci 2012;53:3027-39. [PubMed]
- Terra R, Wang X, Hu Y, et al. To investigate the necessity of STRA6 upregulation in T cells during T cell immune responses. PLoS One 2013;8:e82808. [PubMed]
- Amengual J, Zhang N, Kemerer M, et al. STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum Mol Genet 2014;23:5402-17. [PubMed]
- Zemany L, Kraus BJ, Norseen J, et al. Downregulation of STRA6 in adipocytes and adipose stromovascular fraction in obesity and effects of adipocyte-specific STRA6 knockdown in vivo. Mol Cell Biol 2014;34:1170-86. [PubMed]
- Berry DC, Jacobs H, Marwarha G, et al. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem 2013;288:24528-39. [PubMed]
- Wu L, Ross AC. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res 2010;51:378-87. [PubMed]
- Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J Nutr 2002;132:1160-4. [PubMed]
- Skazik C, Amann PM, Heise R, et al. Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models. J Invest Dermatol 2014;134:1579-88. [PubMed]
- Muenzner M, Tuvia N, Deutschmann C, et al. Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol Cell Biol 2013;33:4068-82. [PubMed]
- Blackfan KD, Wohlbach SB. Vitamin a deficiency in infants: A clinical and pathological study. J. Pediatr 1933;3:679-706.
- Slavotinek AM. Eye development genes and known syndromes. Mol Genet Metab. 2011;104:448-56. [PubMed]
- Chassaing N, Golzio C, Odent S, et al. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum Mutat 2009;30:E673-81. [PubMed]
- Chassaing N, Ragge N, Kariminejad A, et al. Mutation analysis of the STRA6 gene in isolated and non-isolated anophthalmia/microphthalmia. Clin Genet 2013;83:244-50. [PubMed]
- White T, Lu T, Metlapally R, et al. Identification of STRA6 and SKI sequence variants in patients with anophthalmia/microphthalmia. Mol Vis 2008;14:2458-65. [PubMed]
- Gerth-Kahlert C, Williamson K, Ansari M, et al. Clinical and mutation analysis of 51 probands with anophthalmia and/or severe microphthalmia from a single center. Mol Genet Genomic Med 2013;1:15-31. [PubMed]
- Casey J, Kawaguchi R, Morrissey M, et al. First implication of STRA6 mutations in isolated anophthalmia, microphthalmia, and coloboma: a new dimension to the STRA6 phenotype. Hum Mutat 2011;32:1417-26. [PubMed]
- Biesalski HK, Frank J, Beck SC, et al. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am J Clin Nutr 1999;69:931-6. [PubMed]
- Seeliger MW, Biesalski HK, Wissinger B, et al. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci 1999;40:3-11. [PubMed]
- Yahyavi M, Abouzeid H, Gawdat G, et al. ALDH1A3 loss of function causes bilateral anophthalmia/microphthalmia and hypoplasia of the optic nerve and optic chiasm. Hum Mol Genet 2013;22:3250-8. [PubMed]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 2006;133:1901-10. [PubMed]
- Matt N, Dupé V, Garnier JM, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 2005;132:4789-800. [PubMed]
- Quadro L, Hamberger L, Gottesman ME, et al. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 2005;146:4479-90. [PubMed]
- Wassef L, Quadro L. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem 2011;286:32198-207. [PubMed]
- Kim YK, Wassef L, Hamberger L, et al. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem 2008;283:5611-21. [PubMed]
- Wu Y, Li H, Loos RJ, et al. RBP4 variants are significantly associated with plasma RBP4 levels and hypertriglyceridemia risk in Chinese Hans. J Lipid Res 2009;50:1479-86. [PubMed]
- Hu C, Jia W, Zhang R, et al. Effect of RBP4 gene variants on circulating RBP4 concentration and type 2 diabetes in a Chinese population. Diabet Med 2008;25:11-8. [PubMed]
- Qi Q, Yu Z, Ye X, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab 2007;92:4827-34. [PubMed]
- Norseen J, Hosooka T, Hammarstedt A, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:2010-9. [PubMed]
- Moraes-Vieira PM, Yore MM, Dwyer PM, et al. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab 2014;19:512-26. [PubMed]
- Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol 2009;29:3286-96. [PubMed]
- Ziouzenkova O, Orasanu G, Sharlach M, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med 2007;13:695-702. [PubMed]
- Felipe F, Bonet ML, Ribot J, et al. Modulation of resistin expression by retinoic acid and vitamin A status. Diabetes 2004;53:882-9. [PubMed]
- Mercader J, Ribot J, Murano I, et al. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 2006;147:5325-32. [PubMed]
- Mcilroy GD, Delibegovic M, Owen C, et al. Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes 2013;62:825-36. [PubMed]


