Right lobe living-donor hepatectomy—the Toronto approach, tips and tricks
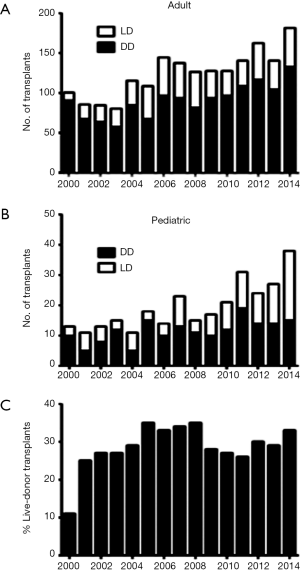
Living-donor liver transplantation (LDLT) is a well-established treatment with outcomes comparable to deceased-donor liver transplantation (DDLT) (1-4). Acceptance of this procedure in North America and Europe, however, has lagged behind Asia. In 2000, we initiated our adult LDLT program, which has become the largest and most active program in North America, accounting for 30% of our total liver transplant activity (Figure 1). In this paper, we discuss the evolution, challenges, and current practices of our adult LDLT program.
Program philosophy
We offer LDLT to all primary liver transplant recipients. This approach is based on our experience showing that LDLT provides similar outcomes to DDLT and significantly reduces waitlist mortality (5). We acknowledge that our philosophy reflects the low rate of deceased donation and high rate of endemic liver disease in Toronto, and may not be applicable to other regions. Indeed, adult LDLT is uncommon in other Canadian centres. Interestingly, despite having an active LDLT program, our annual wait-list mortality rate remains unchanged at 20−25%.
We are cognizant of the ethical tensions of LDLT (6,7), and realize that ongoing success depends entirely on donor safety. We have introduced and incorporated standard operating procedures and policies designed to enhance donor safety including checklists and a disaster plan, and have developed a collaborative multidisciplinary team that strives for continuous quality improvement.
Donor evaluation (Table 1)

Full table
Donor entrance criteria in our program includes age >16 and <60 years, good health, BMI <35, compatible blood group, and capacity to provide informed consent. The evaluation process begins with the completion of a health-screening survey. Once deemed suitable, the donors proceed through a series of investigations that include various blood tests, viral serology, imaging, and consultations with health care professionals from medicine and psychiatry that are independent of the transplant program. In donors >50 years, we obtain a transthoracic echocardiogram and a cardiac perfusion study together with a cardiology consult. In donors with a personal or family history suggestive of a thromboembolic disorder, more extensive coagulation studies are performed under the guidance of a hematologist. Prospective donors meet with a surgeon on two separate visits to discuss the surgery and risks of morbidity and mortality.
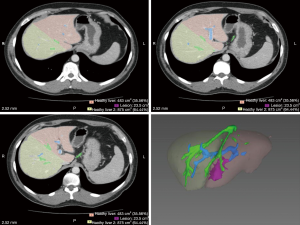
We rely on triphasic computerized tomography (CT) to delineate vascular anatomy and surgical planning of the resection and magnetic resonance cholangiography (MRCP) to define biliary anatomy. We currently employ the Myrian® software package (Intrasense, Paris, France) to estimate graft and donor remnant liver volumes from images captured from CT (Figure 2). We aim to provide recipients with a graft that has an estimated graft to recipient weight ratio (GRWR) ≥0.8 and leave donors with a residual liver volume (RLV) of ≥30%. However, we have successfully used right lobe grafts with GRWR of 0.6 (8).
We obtain liver biopsies in potential donors who have a history of abnormal liver tests and in those with suspected steatosis based on imaging and/or a BMI >30. If the liver biopsy shows micro/macro steatosis >10%, we provide dietary counseling and repeat the biopsy after the donor has completed a successful weight loss program and the imaging studies demonstrate reduction of liver fat. We will not proceed with donation until fat content in the repeat liver biopsy is <10%.
We hold three multi-disciplinary conferences each week to review and discuss potential donors, plan upcoming activity, and review imaging investigations. All members of the team including surgeons, hepatologists, coordinators, nurses and social workers participate in these meetings. Donor exclusion criteria include underlying medical conditions that might increase the risk of complications and death, underlying liver disease, abnormal liver tests, unfavorable vascular and biliary anomalies (discussed further below), insufficient GRWR or RVL, and steatosis >10%. ABO incompatibility has been an absolute exclusion criterion, except for infants <1 year with fulminant liver failure (FLF). Notably, we do not exclude donors who are HBcAb-positive provided their liver biopsy is normal; these grafts are transplanted preferentially into recipients who are HBV-positive or have protective immunity against HBV. About one-third of the potential donors that enter the evaluation process proceed to surgery. This proportion has remained stable over the program’s lifespan, and highlights the enormous amount of resource required to perform LDLT.
Accepted donors undergo weekly review of their imaging studies until the day of surgery. For most donors, this means at least three independent reviews by the entire surgical team. We believe this process minimizes the risk of missing subtle anomalies and provides on-going quality assurance and improvement. In addition, we repeat all blood work to ensure the results are <30 days old on the day of surgery in accordance with Canadian Health Requirements.
Additional considerations
Fulminant liver failure (FLF)
We offer LDLT as an option for patients with FLF. In this situation, the donor evaluation process is expedited and can be completed in 24-36 h. To date, seven adult recipients with FLF have been transplanted successfully with liver donor grafts; long-term patient and graft survival rates are 86% and 86%, respectively, which are similar to those with DDLT for FLF in our centre (9).
Anonymous donors
Since 2005, we have performed 29 LDLT with anonymous non-directed donors. These donors undergo a prescreening process to understand their motivation and psychologic stability before initiating the formal evaluation. We have learned that anonymous and non-anonymous donors derive the same feeling of satisfaction and pride, and experience the same quality of life after donation (10,11).
Donor/recipient anatomy
Arteries
CT imaging demonstrates arterial and venous anatomy accurately in most donors. We pay attention to the origin of the celiac artery to assess stenosis, usually from the arcuate ligament. If it appears to be functionally relevant based on stenosis >70%, presence of post-stenotic dilatation, or prominence of the gastro-duodenal artery, we assess the hepatic arterial waveform by Doppler ultrasonography. If the waveform appears normal during all phases of respiration, we will proceed with donation. Parenthetically, it is also important to know whether celiac artery stenosis exists in the recipient, as this may preclude living-donor transplantation because the absence of donor blood vessels limits arterial reconstruction options for the graft.
We will use right lobe grafts with two arteries, provided the arterial anatomy in the recipient (based on CT) is suitable. The reconstruction method varies with recipient factors and includes arterioplasty to create a single donor artery, separate anastomoses to the right and left hepatic arteries and cystic artery, and an end-to-side anastomosis of one donor artery to the proper hepatic artery.
Intraoperative hepatic arterial dissection in the recipient poses a difficult challenge because of the lack of donor vessels. We have dealt with this problem by mobilizing and rotating the splenic artery towards the liver, using an interposition splenic artery graft between the proximal common hepatic artery or celiac trunk and donor artery, and mobilizing the right gastroepiploic artery. Others centres have used recipient radial artery grafts, but we have yet to employ this solution (12).
Portal vein
Portal vein anomalies alone rarely affect donor candidacy. Grafts with two separate right portal veins can usually be reconstructed with recipient portal vein, often as back-table procedure. We have excluded donors in whom the right anterior vein originates from the distal left portal vein, and those in whom two widely displaced right portal veins were accompanied by bile duct or arterial anomalies. Notably, when a potentially troublesome portal vein anomaly has been identified in the donor, we evaluate the portal venous anatomy in the recipient to determine the feasibility of reconstruction with recipient vessels or banked deceased-donor vessels.
Hepatic veins
Adequacy of hepatic venous outflow is a key determinant of graft and patient outcome (13). Perhaps the most contentious issue in right lobe-LDLT is whether the middle hepatic vein should be taken with graft. In the past, we were strong advocates for routine middle hepatic vein revascularization to reduce the risk of graft congestion (14). Over the past 5 years, however, we have shifted towards a more selective approach, limiting MHV removal to grafts in which there are large segment V and VIII venous tributaries and minor contributions from segment IV, particularly if the GRWR is <0.8 and the recipient has evidence of severe portal hypertension. Large segment VIII veins can usually be connected to the RHV or MHV directly or to the inferior vena cava via a recipient left portal vein graft (15). We have not perceived an adverse effect in our recipients using this approach, and found that graft congestion, when it occurs, can be treated effectively by reducing portal venous inflow with splenic artery ligation or splenectomy. We have no experience with the use of PTFE grafts to drain segment V and VIII veins, as reported by others (13).
Bile ducts
Progressive improvement in MRI technology has enabled accurate depiction of biliary anatomy in most donors (16). We believe this is an important component of their assessment to reduce the risk of a “no-go” hepatectomy (17). We avoid right lobe donors with ≥3 right hepatic ducts and those with segment IV ducts that enter the right anterior or posterior hepatic ducts above the main right and left duct confluence.
Donor surgery
Patient position on the operating table requires special attention to avoid neuropraxia injuries from nerve compression and elongation during the surgery. Our standard approach involves tucking both arms beside the body, ensuring good padding between the arms and retractor side-posts, and hourly reassessment of the arms by nursing and anesthesia staff. For large patients in whom tucking the arms is not feasible on a standard operating table, we prefer to add a side extension to the table rather than extending an arm.
We use a small hockey-stick incision in most patients (18), although a midline incision is feasible in some right lobe donors, and the Thompson Retractor®. After inspection and palpation of the liver, the right lobe of the liver is fully mobilized by dividing the right triangular ligament and ligating caudate lobe vein tributaries to the inferior vena cava. Segments VI and or VII venous tributaries >8 mm in diameter are preserved for re-implantation in the recipient. The right hepatic vein is encircled with an umbilical tape, which is used during the parenchymal transection for the “hanging manoeuvre” (19).
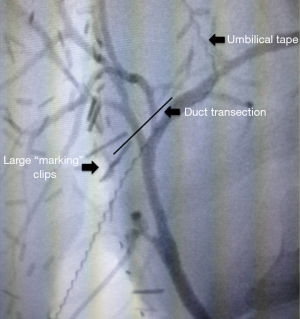
We next mobilize the gallbladder off the liver bed, lower the hepatic plate, and perform a cholangiogram to verify biliary anatomy. We introduce the cholangiogram catheter into the cystic duct or gallbladder, and use fluoroscopy to monitor the dye as it flows into the hepatic ducts. We find that a large metal clip placed beside the right hepatic duct helps us define the bile duct transection line later in the procedure (Figure 3). In 2% of donors, the cholangiogram reveals unexpected biliary anomalies that preclude proceeding with the liver resection (17).
We next dissect and encircle the right hepatic artery(s) and portal vein(s), avoiding the bile duct and it’s adventitia as much as possible. The right hepatic artery is usually mobilized proximal to the cystic artery branch, which can be incorporated into a branch-patch angioplasty for the anastomosis in the recipient. We also pay particular attention to the course of the anterior branch of the right hepatic artery, because it sometimes deviates leftward and can be mistaken for a left lobe branch. One or two small caudate branches from the right portal vein are ligated routinely to mobilize the right portal vein. Clear identification of left portal vein and its relationship to the main portal vein is essential in grafts with two right portal veins.
After completing the portal dissection, we score the liver surface with cautery to mark the transection line, which runs from the right side of MHV to the mid-point the gallbladder fossa; the transection line in gallbladder fossa deviates into the base of segment IVb to help ensure parenchyma and plate tissue remains around the right hepatic duct. Over the past 12 years, we have used the Hydro-Jet Dissector (ERBE, Teubingen, Germany) to transect the liver. Bleeding is controlled mainly with monopolar electrocautery without inflow occlusion, supplemented with hemostatic clips, silk ties, and occasionally staplers for larger portal structures and hepatic vein tributaries. A red blood cell scavenging device is used routinely. Segment IV and V veins serve as the main guides that lead to the distal MHV. Once the MHV has been well defined, segment V or IV vein tributaries are ligated depending on whether we plan to leave or remove the MHV, respectively; transection continues along the course of the MHV, which is usually “skeletonized” as we proceed. We correlate intraoperative observations with the preoperative CT images to guide the transection line, and rarely use intraoperative ultrasound. Segment VIII veins are ligated or preserved for revascularization when their diameter exceeds 1 cm. As the transection approaches the portal plate, we veer leftward into the base of segment IV.
Before dividing the right bile duct, we may repeat a cholangiogram to ensure that the marking clip placed beside the right bile duct is in good position. Notably, we avoid direct dissection of the bile ducts and rely mostly on the marking clip to guide the point of division (5). The right biliary plate and bile duct are divided sharply with scissors, maintaining hemostasis with suture ligatures of 6-0 prolene. The duct lumen is probed extensively to verify the exact location of the left and common ducts. In most cases, we divide at least one caudate duct, which is suture ligated with 6-0 PDS.
We administer of 2,500−5,000 U of heparin systemically just prior to graft removal. The hepatic artery is ligated with silk ties and clips. The right portal vein is secured and divided with an endovascular TA stapler or a small satinsky vascular clamp. Finally, the right hepatic vein is secured and divided with an endovascular TA stapler. We flush the right portal vein with 1−1.5 L of ice-cold histidine-tryptophan-ketoglutarate (HTK) solution, and occasionally retrograde flush the hepatic veins. The hepatic artery(s) and duct(s) are also flushed using a small catheter and syringe. The liver is packed on ice and transferred to the recipient operating room.
The stump of the right hepatic duct(s) is closed with 6/0 polydioxanone. A final cholangiogram is performed to verify duct integrity. Before the abdomen is closed the falciform ligament is repaired to support the liver and prevent rotation, and “TAP” catheters are inserted under direct vision for post-op pain control (20). Drains are not employed routinely.
Perioperative and postoperative care
Prophylactic antimicrobial (cefazolin, metronidazole) and anti-deep vein thrombosis (DVT; heparin 5,000 U subcutaneously) therapy is initiated immediately before surgery. Pneumatic compression stockings are also used routinely to reduce the risk of DVT. Postoperatively, donors are managed in an acute care unit for the first 48 h and then transferred to the regular transplant unit. Complete blood counts, coagulation profile and serum liver tests are monitored daily. Patients are given phosphate infusions routinely until tolerating diet and then are transitioned and discharged home on oral phosphate as required. DVT prophylaxis is continued with heparin 5,000 U subcutaneously twice daily and sequential compression stockings while in the hospital and low molecular weight heparin (dalteparin sodium injection; Pharmacia, Canada) 5,000 U subcutaneously daily for 6 weeks after discharge.
All patients undergo routine Doppler ultrasound of the abdomen on the third postoperative day to assess vessel integrity and flow. Donors are followed up with routine laboratory investigations and abdominal ultrasound in the outpatient clinic.
Right lobe-LDLT experience
Between April 2000 and May 2014, we performed 469 right-lobe LDLTs. In the same study period only six left lobe grafts were used in adult-to-adult transplants. Median donor age was 37 years (range, 17−61 years) with 9% >55 years; 53% were female; and mean BMI was 26±5 kg/cm2. The donors were blood relatives (n=289), spouses and friends (n=164), or anonymous (n=16). The MHV was included in 30% of the grafts. Median length of stay was 6 days (range, 4−17 days). There have been no perioperative donor deaths. One donor died from metastatic esophageal cancer 1 year after donation. The overall complication rate was 12% (57 patients), with 91% of the complications occurring within the first 30 days. Eleven patients (2%) suffered a major complication (Dindo-Clavien ≥3b); the rest were minor (i.e., Dindo-Clavien ≤2). Serious complications included anaphylaxis during the anesthetic induction (n=1), pulmonary embolism (n=4), prolonged ventilation from fluid overload (n=1), narcotic over-dosage (n=2), and bile injury during the resection that required repair with a roux-en-Y of jejunum (n=2). Incisional hernias were repaired surgically in 5 patients >1 year after donation.
The mean age of the recipients in this cohort was 52±11 years and 62% were male. The causes of liver failure included HCV (33%), alcohol (15%), primary sclerosing cholangitis (13%), primary biliary cirrhosis (8%), NASH (6%), and HBV (6%). In 27% of the recipients, there was an associated hepatocellular carcinoma. The mean medical MELD score at the time of transplantation was 17±7. All living donor-recipients are placed on the wait-list for deceased-donor transplantation; the median time between wait-listing and LDLT was 118 days (1−2,736 days). The incidence of major complications (> Clavien-Dindo 3b) within 30 days of the LDLT was 24% and the incidence of hepatic artery thrombosis was 3%. The median ICU and hospital stay was 1 day (1−159 days) and 12 days (1−160 days), respectively.
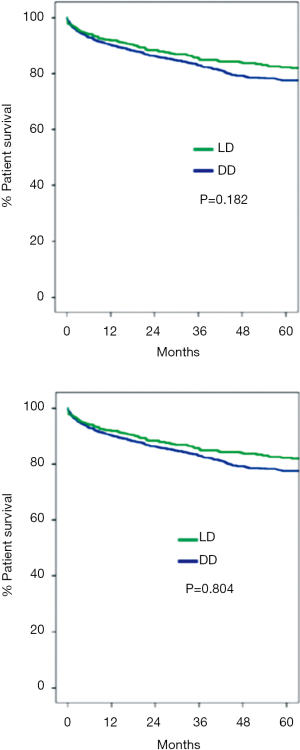
Graft and patient survival at 1-, 3- and 5-years was 90%, 83%, 80% and 92%, 85% and 82%, respectively. There were no significant differences in the patient and graft survival rates among recipients of LDLT and DDLT over the 15-year study period (Figure 4). The same immunosuppression protocol was used in both groups of recipients: induction therapy with thymoglobulin (Genzyme Canada) or basiliximab (Novartis, Canada) was used selectively in recipients with renal dysfunction; and maintenance therapy consisted of tacrolimus (Astellas Canada) in most patients and cyclosporine (Novartis, Canada) in patients infected with hepatitis C virus. As in other centres, we have observed that LDLT recipients experience a higher rate of biliary complications (13). Over time, there has been a significant decrease in the rates of early bile leaks; however, the 5-year Kaplan-Meir stricture rate remains high at 18%. Fortunately, most of these strictures can be managed with percutaneous/endoscopic dilatation and stenting or surgical revision. So far, only 4 patients have required liver retransplantation because of a refractory biliary stricture.
Discussion
LDLT has evolved into a highly effective therapy for patients with end-stage liver failure. Through the collective world experience over the past 20 years, many of the technical challenges of LDLT have been solved or mitigated, resulting in excellent long-term outcomes. For example, “small-for-size syndrome”, a cause of early graft failure, can generally be avoided through preoperative planning and technical maneuvers that optimize venous outflow and portal inflow. Moreover, LDLT enables optimal timing of transplantation, minimizing the risk of pre-transplant deterioration and death, and facilitating adjuvant therapy for hepatocellular carcinoma and hepatitis C.
Our program embraced LDLT as a solution for the stark disparity between organ need and availability of deceased donors. Notwithstanding the success and growth of our program, we remain mindful of the tremendous sacrifice of our living donors who must not only face the obvious risks from surgery, but also endure the financial burdens from time off work, relocation, and travel. Through lobbying efforts, we convinced the Government of Ontario to offset some of the costs of donation with a reimbursement program that began in 2007. This program has helped many donors, although it still does not cover all their costs, nor does it help all donors who come from other Canadian provinces if the recipient is non-Ontarian. Providing financial support for patients who develop a serious complication that takes time to resolve is a particularly important challenge. Clearly, more needs to be done. We also need a deeper understanding of the long-term effects of living donation. Detailed quality-of-life studies by the A2ALL group raise concerns that some donors may experience adverse effects that often escape attention in most reports.
Since the inception of our program, we have transplanted right-lobe grafts in the vast most of our adult recipients. We are cognizant of the potential merits of left lobe grafts with respect to donor safety (21,22). Our enthusiasm for these grafts in adults, however, is tempered by the increased risk of small-for-size syndrome in the recipient. Diverting portal-systemic vein shunts to reduce portal inflow in the graft may offset this risk (23,24), although judging the optimal size of the shunt represents a real challenge.
We continue to explore other options that can expand the deceased donor pool. Last year, death-after-Cardiac (DCD) donors accounted for 12% of our adult activity. We have also embarked on new ex-vivo organ preservation strategies to improve liver function, and hopefully, increase the utilization of older and steatosis grafts. It is notable, however, that the proportion of LDLTs in our program has remained constant at 30−35%, despite our efforts to maximize DDLT.
As in many regions of world, we have seen an inexorable increase in the age of deceased donors with a corresponding increase in donor co-morbidity, suggesting that there may be a finite limit to the growth of DDLT. It therefore seems likely that LDLT will have to increase in North America and Europe to meet the increasing demand for liver transplantation. Whether LDLT should be offered by all centers or limited to high-volume centres with greater expertise is a question that needs to be considered carefully. There is evidence of a significant learning curve with LDLT and superior outcomes are generally achieved at higher-volume programs (25). Thus, current evidence suggests that a centralized delivery model is more likely to ensure donor safety and excellent recipient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pomposelli JJ, Verbesey J, Simpson MA, et al. Improved survival after live donor adult liver transplantation (LDALT) using right lobe grafts: program experience and lessons learned. Am J Transplant 2006;6:589-98. [PubMed]
- Maluf DG, Stravitz RT, Cotterell AH, et al. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant 2005;5:149-56. [PubMed]
- Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant 2008;8:2569-79. [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann Surg 2006;243:404-10. [PubMed]
- Shah SA, Levy GA, Greig PD, et al. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant 2007;7:998-1002. [PubMed]
- Gordon EJ. Informed consent for living donation: a review of key empirical studies, ethical challenges and future research. Am J Transplant 2012;12:2273-80. [PubMed]
- Gordon EJ. Ethical considerations in live donor transplantation: should complications be tolerated? Curr Opin Organ Transplant 2013;18:235-40. [PubMed]
- Selzner M, Kashfi A, Cattral MS, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl 2009;15:1776-82. [PubMed]
- Goldaracena N, Spetzler VN, Marquez M, et al. Live donor liver transplantation: a valid alternative for critically ill patients suffering from acute liver failure. Am J Transplant 2015;15:1591-7. [PubMed]
- Franks I. Transplantation: Anonymous living liver donors--outcomes and motivations. Nat Rev Gastroenterol Hepatol 2010;7:592. [PubMed]
- Reichman TW, Fox A, Adcock L, et al. Anonymous living liver donation: donor profiles and outcomes. Am J Transplant 2010;10:2099-104. [PubMed]
- Lin TS, Yang JC, Chen CL. Hepatic artery reconstruction using radial artery interposition graft in living donor liver transplantation. Transpl Int 2013;26:e28-30. [PubMed]
- Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015;15:17-38. [PubMed]
- Cattral MS, Molinari M, Vollmer CM Jr, et al. Living-donor right hepatectomy with or without inclusion of middle hepatic vein: comparison of morbidity and outcome in 56 patients. Am J Transplant 2004;4:751-7. [PubMed]
- Cattral MS, Greig PD, Muradali D, et al. Reconstruction of middle hepatic vein of a living-donor right lobe liver graft with recipient left portal vein. Transplantation 2001;71:1864-6. [PubMed]
- Kim RD, Sakamoto S, Haider MA, et al. Role of magnetic resonance cholangiography in assessing biliary anatomy in right lobe living donors. Transplantation 2005;79:1417-21. [PubMed]
- Guba M, Adcock L, MacLeod C, et al. Intraoperative 'no go' donor hepatectomies in living donor liver transplantation. Am J Transplant 2010;10:612-8. [PubMed]
- Dixon E, Sahajpal A, Vollmer CM Jr, et al. Multipurpose extended subcostal incision for hepatobiliary surgery. Am J Surg 2004;187:128-30. [PubMed]
- Belghiti J, Guevara OA, Noun R, et al. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg 2001;193:109-11. [PubMed]
- Young MJ, Gorlin AW, Modest VE, et al. Clinical implications of the transversus abdominis plane block in adults. Anesthesiol Res Pract 2012;2012:731645.
- Roll GR, Parekh JR, Parker WF, et al. Left hepatectomy versus right hepatectomy for living donor liver transplantation: shifting the risk from the donor to the recipient. Liver Transpl 2013;19:472-81. [PubMed]
- Reichman TW, Sandroussi C, Azouz SM, et al. Living donor hepatectomy: the importance of the residual liver volume. Liver Transpl 2011;17:1404-11. [PubMed]
- Botha JF, Langnas AN, Campos BD, et al. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl 2010;16:649-57. [PubMed]
- Troisi R, Ricciardi S, Smeets P, et al. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant 2005;5:1397-404. [PubMed]
- Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg 2005;242:314-23, discussion 323-5. [PubMed]


