Robotic liver surgery: technical aspects and review of the literature
Introduction
Minimally invasive surgery for liver resections represents an accepted alternative to open techniques for selected cases. Currently, laparoscopy is used as the only standard approach for resection of the anterior segments (II to VI) and left lateral sectionectomies (1-8).
Advantages, such as less estimated blood loss and postoperative pain, lower morbidity, shorter hospital stay and improved cosmesis, have been well established in current literature (1,7,9-11). Oncologic laparoscopic liver resections have also proven to be feasible and safe when performed in expert hands, with oncologic outcomes that are equivalent to traditional open surgery in terms of margin infiltration and local recurrence (1,2,5,10,12-17). In liver surgery, laparoscopy presents some peculiar advantages as the preservation of the abdominal wall from large subcostal incisions. This translates into better postoperative diaphragmatic function with less respiratory complications, better venous drainage in cirrhotic patients, less postoperative ascites and reduced pain. There are also long term advantages like less risk of incisional hernias and peritoneal adhesions. Furthermore, pneumoperitoneum has been shown to decrease oozing from the transection line with a positive impact on the overall blood loss.
Nonetheless, laparoscopy has some disadvantages that hinder its wider adoption, mainly in major hepatic resections and complex cases (3,6,7,18). Limited degrees of motion of the instruments, unstable camera platform, two-dimensional vision and poor ergonomy are all factors that increase the difficulty of the procedure.
It is interesting to note that in 2008 the Louisville consensus conference limited or contraindicated the role of laparoscopy for major hepatectomies or when biliary reconstruction is needed (extended hepatectomies), when the lesion is adjacent to major vessels or in close proximity of the liver hilum. After six years, the recommendations by the last consensus conference, held in Morioka, still confirms the same indications due to a lack of evidence to generate new recommendations (19). No specific recommendations were made on robotic liver surgery, even though the studies present in current literature suggest that outcomes are not inferior to other techniques (19).
Robotic assisted liver surgery
Robotic technology, designed to overcome some of the limitations of laparoscopy, is gaining interest in this field as proven by the constantly growing number of reports in the literature. The stability of the robotic platform, combined with the three-dimensional, magnified high-definition vision, increased degrees of freedom of the instruments and tremor filtering provide higher dexterity to the surgeon and allow for the same movements of open surgery. Precise dissection and suturing is possible, even in narrow operative fields, allowing for easier dissection of the hepatic hilum, fine lymphadenectomy, biliary reconstruction even with small bile ducts and more effective bleeding control (3,4,6,20-22).
The safety and feasibility of this approach has been clearly demonstrated (1,3,4,6,10,11,20,21,23-28). The promising results suggest that Robotics has the potential to expand the indications to more complex cases such as major hepatectomies, extended hepatectomies with biliary reconstruction and difficult segmentectomies of the posterior-superior segments (4,7,22,24). Furthermore, the digital interaction with the target facilitates many potential innovations like the recent near-infrared fluorescence and the soon to come image guided surgery and augmented reality.
Near-infrared fluorescence in robotic liver surgery
The robotic platform provides additional advantages, like integrated near-infrared fluorescence imaging. Indocyanine green (ICG) is a non-toxic fluorophore that appears green when stimulated by near-infrared light. It is approved by the Food and Drug Administration (FDA) and has been used in medicine for over 40 years (29,30). Arteries and veins can be visualized 5−60 seconds after intravenous injection. ICG then accumulates in the liver and is secreted in the bile 45−60 minutes later, allowing for visualization of the biliary structures. Recognition of vascular and biliary anatomy is important in hepatic surgery, especially during dissection of the hilum. It could help to decrease intraoperative complications, especially in the presence of anatomical variations. In our practice, ICG fluorescence is used in all hepatobiliary procedures (31,32). Moreover, this technique has interesting perspectives for differentiation of hepatic lesions based on their vascular pattern. Well-differentiated hepatocarcinomas (HCC) are hyperfluorescent, while poorly-differentiated HCCs and colorectal metastases are hypofluorescent (30,33). In adjunction to preoperative imaging, this method could increase accuracy of lesion detection, or even distinction between benign and malignant masses. Future technological advancements will include new fluorophores conjugated to monoclonal antibodies, leading to a type of ‘fluorescence-guided’ surgery, real-time in vivo microscopy for evaluation of resection margins, as well as accurate identification of metastatic versus normal lymph nodes (31).
Limits of robotic liver surgery
One of the limits of robotic HPB surgery is the need for specialized training, not only for the primary surgeon, but also for the assistant surgeon and OR nurses, although in some cases, the learning curve for specific robotic procedures has proven to be shorter than the laparoscopic equivalent (17). Moreover, hepatobiliary surgeons, at the beginning of their robotic learning curve, might have a limited number of simple cases that could be used as a training model. Simulation and virtual-reality surgical training are promising, but are still under development and require validation (34,35). The robotic dual console is a teaching tool that could help accelerate proficiency (36). Another problem, more specific to liver surgery, is that currently there are only a limited number of robotic instruments for parenchymal transection. The harmonic shears are very efficient in cutting and coagulating, if properly used, but do not have all the degrees of freedom of other robotic tools. This limitation requires some adjustments during the procedure to align the instrument with the section line.
Technique: robot-assisted right hepatectomy
Patient positioning and trocar placement
The patient is in the supine position, with parted legs in 20 degree reverse-Trendelemburg. The assistant surgeon is positioned between the patient’s legs. Pneumoperitoneum is achieved with the Veress needle, preferably at the left upper quadrant. A 10−12 mm trocar is then placed above the umbilicus and is used as an assistant port during the first steps of the procedure (retraction, suction/irrigation, stapling) and as an operative port during the parenchymal transection. A 12-mm trocar is placed in the right midclavicular line (10 cm from the assistant trocar) to act as a camera port. The trocar for the first robotic arm is placed in the left midclavicular line (10 cm from the assistant trocar) and the trocar for the second robotic arm is placed in the right anterior axillary line (10 cm from the optical trocar). The third robotic arm port is placed in the left anterior axillary line and is used for retraction. The body habitus of each patient needs to be assessed, since adjustments may be needed in order to avoid arm collision and achieve optimal exposure. At this point, a diagnostic laparoscopy is performed in order to exclude the presence of metastases. An intraoperative ultrasound is also performed in order to have a better understanding of the size, number and location of the lesions, as well as to detect any contralateral nodules. The robotic cart is brought into the surgical field, coming from the patient’s head, and the arms are docked (Figure 1).
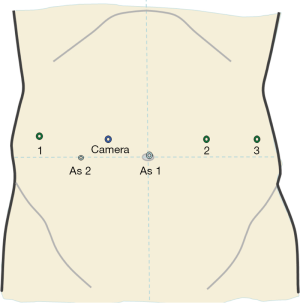
Surgical procedure
Three steps are clearly defined. The first step is the dissection of the hepatic hilum. First, a retrograde cholecystectomy is performed. The hepatic pedicle is dissected using a combination of monopolar hooks and bipolar forceps. The right hepatic artery is dissected first and then sectioned between prolene sutures. The portal vein is completely dissected and selective stitches or ligatures are applied on the small posterior branches for segment I. The right portal vein is then divided between robotic clips and sutured with either 4-0 or 5-0 prolene. An extrahepatic dissection of the right bile duct should be performed only when the anatomy is clear and confluence of the biliary ducts is low. In such a case, when the hilar plate is lowered, the right hepatic duct is isolated and transected approximately 1 cm from the bifurcation. In other cases, the division of the right hepatic duct should be intrahepatic, during the transection of the parenchyma. ICG fluorescence can be easily used at any point and can help identify the biliary anatomy.
The second step of the procedure is the hepatocaval dissection. The falciform ligament and coronary ligament are sectioned. The lateral reflection of the peritoneum is dissected along the hepatocaval plane. The third arm is used to lift the inferior surface of the right lobe to expose the inferior vena cava (IVC). The accessory hepatic veins are sectioned between ligatures or transfixed stitches of prolene. Robotic clips can also be placed for accessory hepatic veins of minor caliber or to further secure the proximal ligature. The dissection of the IVC should proceed until the inferior aspect of the right hepatic vein is visible, close to the diaphragm. In selected cases, a true ‘hanging maneuver’ can be achieved.
Transection of the liver is the last step of the operation. Parenchymal transection should follow the ischemic demarcation line and start at the anterior aspect of the liver, along the cholecysto-caval line. The central venous pressure (CVP) should be lowered to less than 5 mmHg in order to reduce blood loss (37). The 2/0 prolene stay sutures are placed along the anterior border of the liver in order to retract the left lobe and expose the section line. Bipolar forceps and robotic harmonic shears are the main tools for the parenchymal transection. The transection is performed layer by layer, starting from the cortical aspect of the liver. It is important to proceed this way keeping the entire section line always under control. Because the harmonic shears lack articulating ability, the first arm is shifted into the midline 12 mm port with a trocar in trocar technique. This allows for better alignment of the instrument with the section line. Minor bleedings can be controlled using bipolar cautery or harmonic shears, while larger vessels should be selectively sutured with prolene stitches. After the sub-cortical aspect of the liver is sectioned, the transection proceeds towards the core of the liver parenchyma. This portion of the liver includes bigger vessels, like venous branches coming from segments V and VIII, and directed to the middle hepatic vein. At this point, laparoscopic staplers are used for the parenchyma and the intracapsular control of the right hepatic vein. The bed side assistant surgeon has a key role at this stage. The liver is then completely mobilized by sectioning the remaining peritoneal attachments. The raw surface of the remaining liver should then be examined for bleeding and bile leak. Raising the CVP helps with checking the effectiveness of the hemostasis. Fluorescence can be used to detect bile leaks from the hepatic remnant, using irrigation. At the end, fibrin glue can be applied to the remaining surface as a sealant. Although some authors perform the Pringle maneuver to prevent excessive blood loss during hepatic resection, we do not find it necessary (38,39). Finally, the specimen is placed in an endoscopic bag and extracted through a small Pfannenstiel incision or through the site of a previous scar, if present. We normally place two closed suction drains in the subhepatic and subdiaphragmatic area. The robotic cart is removed from the operative field, pneumoperitoneum is stopped and the trocars are extracted under direct laparoscopic vision.
Technique: robot-assisted left hepatectomy
Patient positioning and trocar placement
The patient positioning and trocar placement are similar to the right hepatectomy. The third arm is generally kept on the left side but, in few cases, is moved on the far right to allow for more space for the operative arms on the left.
Surgical procedure
The operation begins with sectioning the round, falciform and left triangular ligaments in order to mobilize the left lobe of the liver. The left hepatic artery is identified, along the left side of the hepatoduodenal ligament, and is then dissected and sectioned between sutures after confirming the correct interpretation of the anatomy. At this point, the left portal branch is identified and sectioned between ligatures. The left hepatic biliary duct is located just above the left portal vein and divided between robotic clips and sutures. We always perform an extraparenchymal dissection of these structures.
The principles of parenchymal transection are equivalent to right hepatectomy. The transection should proceed layer by layer using the harmonic shears from the cortical aspect of the liver towards the core of the parenchyma. Stay sutures should be placed on the left side of the transection line and held by the third robotic arm in order to provide exposure of the hepatic section line. Bleeding can be managed with bipolar cautery, harmonic shears and/or selective sutures and robotic clips. The residual parenchyma and left hepatic vein are divided using a laparoscopic vascular stapler. The surface of the remnant is checked and the specimen is extracted, as described previously. Two closed suction drains are placed around the resected area.
Technique: robot-assisted segmentectomies
Patient positioning and trocar placement
The patient positioning and trocar placement can be variable depending on the segments to be resected. Trocars will be positioned very high subcostal and lateral for the posterior superior segments or closer to the transverse umbilical line for the anterior segments shifting toward the left or the right depending on the lesion location. The basic rule is to create an adequate triangulation with enough space in between the ports. The assistant ports can be placed slightly caudally from the robotic ports line to allow for more room for movements outside the abdominal cavity. Due to the limited degree of freedom of the Harmonic, correct positioning of the instrument is critical in order to follow the section line. Sometimes this might require a switch of the instrument in between the left and right operative arm.
A laparoscopic exploration of the abdominal cavity and an intraoperative laparoscopic ultrasound are performed. Those are crucial in order to assess the lesions and their relationship with the anatomical structures of the liver and plan for adequate margins.
Surgical procedure
In our experience the Pringle maneuver has been rarely used but when there is a need to secure more control on the liver inflow, the hepatic pedicle is prepared and a tourniquet is created using an umbilical tape.
The main tool used for parenchymal transection is the robotic harmonic shears. The correct docking of the robotic arm holding the instrument is crucial in order to align the harmonic with the section line. In some cases, the robotic arm can be shifted in one of the assistant ports using a trocar-in-trocar technique. Transection is performed layer by layer, starting from the surface and proceeding towards the core. The segment/mass should be retracted very gently in order to avoid rupture of the lesion, this can be achieved with stay sutures or using the fourth robotic arm with small sponges. Once the resection is completed, hemostasis is perfected with the robotic bipolar forceps and selective stitches can be applied if needed.
Review of the literature
After searching the PubMed database with the MeSH terms: ‘robotic liver resection’, ‘robotic hepatectomy’, ‘robotic hepatic resection’ and ‘robotic liver’, we selected 12 major series that included more than ten cases with a total of 348 patients undergoing a robotic liver resection. All articles that were taken into consideration report intraoperative and postoperative outcomes. The majority of the studies also include the resection margin status (R0−R1). Two articles did not make a distinction between major and minor hepatectomies in reporting their results (19,22). We excluded two articles that reported the authors’ initial experience, since the same cases were included in larger series that were later published by the same authors (23,24,39,40).
In our experience, all segments are amenable to resection. Some authors have reported that robot-assistance especially facilitates the resection of lesions located in the posterior/superior segments (38,39). Nonetheless, even though the resection of such lesions could be easier with the robotic technique instead of the laparoscopic, it can still prove to be very challenging.
Reviewing the current literature, we found that indications for robotic liver surgery included both malignant and benign disease; with the first being the most frequent, exceeding 70%. HCC was the most common indication among the neoplasms (51%), followed by colorectal metastases (35%). Of the benign lesions, 30% were hemangiomas, 20.5% focal nodular hyperplasia (FNH) and 13.7% intrahepatic duct stones.
Contraindications to the robotic approach generally included invasion of major hepatic vessels, and extension into the diaphragm, even though the latter could still be feasible in selected cases. There is no predetermined limit regarding the size of lesions, but very bulky tumors can be difficult to resect.
Major hepatectomies
Major hepatectomy is a complex procedure that requires advanced surgical knowledge and skills. Although minimally invasive resections of the liver are performed more frequently in past years, major resections are still a minority of those cases. In fact, the cases described in current literature are 149, representing 47% of total robotic liver cases (11).
The largest series of robotic hepatectomy was reported by Giulianotti et al. (4) in 2011 with a total of 70 hepatic resections, of which 27 were major hepatectomies. The most common procedure was right hepatectomy (n=20), followed by left hepatectomy (n=5). The most frequent indication was malignancy (60%). The mean operative time was 313 minutes with an estimated blood loss of 150 mL and a transfusion rate of 22%. The conversion-to-open rate was 3.7%. Overall morbidity was 29.6% with zero mortality. Resection margins were negative in all cases.
Our most recent experience includes 60 cases of major hepatectomy. The transfusion rate has decreased to 15% and the rate of significant postoperative complications is currently 10%. The conversion rate is 11%, reflecting the increased complexity of cases being performed. The most common reason for conversion-to-open in our series was very bulky lesions and unclear tumor margins.
Recently, Spampinato et al. (41) performed a retrospective study comparing the perioperative outcomes of robot-assisted major hepatectomy and laparoscopic major hepatectomy in four Italian centers. A total of 50 major hepatectomies were considered, including 25 robotic and 25 laparoscopic resections. The mean robotic operative time was 430 min, with a median EBL of 250 mL, comparable to laparoscopy. Intermittent pedicle occlusion was required only in the laparoscopic group (32% vs. 0%). The ability to control bleeding effectively during parenchymal transection allows for avoidance of intermittent pedicle occlusion.
In 2014, Tsung et al. (7) performed a matched series comparison of surgical and postsurgical outcomes between robotic (n=57), laparoscopic (n=114), and open hepatic resections (n=21). The robotic hepatectomy series included 21 major liver resections. The authors considered the resection of 4 or more liver segments as a major resection. Mean operative time was 330 minutes with a mean EBL of 200 mL. Transfusion rate was 7% and conversion-to-open occurred in 19% of cases. Morbidity rate was 24%. Mortality and rate of positive resection margins was zero. A statistically significant difference was seen when comparing the EBL of robotic versus open surgery, as well as in the length of hospital stay. The authors concluded that laparoscopic and robotic liver resections are comparable, even with no demonstration of clear superiority of the robotic approach in terms of outcomes.
Wu et al. (22) reported 52 robotic hepatic resections, including 20 major hepatectomies. In their paper, they analyzed the results of 38 procedures performed for HCC, with no distinction between major and minor. They compared these cases to 41 laparoscopic cases done in the same center. Conversion to open, morbidity, and mortality rates were comparable in the two groups. However, their results showed a longer operation time (380 vs. 227 mL) and greater blood loss (325 vs. 480 mL) in the robotic group. Interestingly, they describe that the use of the robotic approach lead to a twofold increase in minimally invasive liver resections, as well as an increase of the percentage of cases of HCC performed in such a manner.
Choi et al. (21) published the results of 20 major liver resections. The mean operative time was 621 min, with a mean blood loss of 478 mL and 15% transfusion rate. The conversion rate was 10% and the overall morbidity rate was 40%. The authors recorded the operative time as a tool to assess their learning curve in left hepatectomies. They found a clear cutoff point after the seventh case, where the total operating and console time began to gradually decrease.
In a smaller series, Lai et al. (23) described 42 liver resections, of which ten were major hepatectomies. The authors did not differentiate the results between major and minor liver resections, but did observe favorable results with the robotic technique, including a 7.1% complication rate. In their experience, major anatomical dissection was feasible, and with low blood loss, due to the ability to perform accurate extraparenchymal dissection of the portal pedicles and hepatic veins before transection.
The results of the most important series of major hepatectomies are summarized in Table 1.
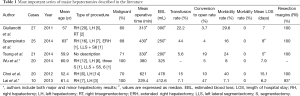
Full table
Extended liver resections have also been described in the literature. In 2010, Giulianotti et al. were the first to describe a case of extended right hepatectomy with biliary reconstruction for hilar cholangiocarcinoma (42). The series, published in 2011, also included two cases of right trisectionectomy (4). A case report of robotic left hepatectomy with revision hepaticojejunostomy has also been published by Chen et al. (43). Spampinato et al. also included a case of extended right hemihepatectomy and Ji et al. described one case of left hemihepatectomy with caudate lobe resection (25,41). Specific results regarding these individual cases have not been reported since they are part of the larger series previously described in this review.
The number of major hepatectomies reported in the literature is still somewhat limited, although steadily increasing. The overall data suggests that this technique is comparable to both open and laparoscopic surgery in terms of perioperative and postoperative outcomes, as well as oncologic efficacy. Complex procedures, such as extender liver resections, are made feasible by the intrinsic advantages of the robotic system. Still, this type of complex procedure should be performed by skilled surgeons, specialized in both robotic and hepatobiliary surgery, while maintaining the correct indications.
Minor hepatectomies
Worldwide, the most common liver procedure performed using the robotic approach is minor hepatectomy. Anatomic and non-anatomic segmentectomies are the most frequently performed (28.6%), followed by left lateral sectionectomies (13%) and bisegmentectomies (9%).
In our 2011 series, we described the results of 43 minor hepatectomies (4). The most common resection was segmentectomy (16 cases), bisegmentectomy (10 cases) and left lateral sectionectomy (9 cases). The most frequent indication for surgery was malignancy (60%). The mean operative time was 198 min, with an EBL of 150 mL and a 20.9% transfusion rate. Conversion to open occurred in 7% of cases. The mortality was zero and the overall morbidity rate was 16%. Resection margins were all negative. Our most recent experience includes 77 cases of minor resections. The transfusion rate is 6.5% and the conversion rate has also decreased to 5%. The overall morbidity rate is currently 9%.
Troisi et al. (24,39) compared 223 patients who underwent laparoscopic liver resection with 38 patients who had a robotic hepatic resection. The most common resection was segmentectomy or wedge resection (15 cases) and the indication was a malignant tumor in 70% of the cases. The mean operative time and EBL was 271 and 330 mL. Mortality was zero and the morbidity rate was 12.5%. The conversion-to-open surgery was 20%. A negative resection margin was achieved in 92.5% of patients. In their experience, the robot-assisted technique allowed for a more conservative approach, with a greater number of lesions that can be resected, preserving hepatic parenchyma and avoiding major hepatic procedures. Also, in their first experience published in 2011, the same authors concluded that robot-assistance facilitates the resection of lesions localized in the posterior/superior segments, as well as lesions in contact with major liver vessels (39).
In a recent article, Tsung et al. performed a matched comparison of patients undergoing robotic and laparoscopic liver resection (7). Fifty-seven patients underwent robotic hepatectomy and 114 laparoscopic hepatectomy. There were 36 cases of minor hepatectomy. The median operative time was 198 min, with a median EBL of 285 mL. The transfusion rate was 2.9%. No conversions-to-open occurred in this series. Mortality was zero and morbidity was 17%. The resection margins were negative in 93% of cases.
In 2013, a prospective evaluation of robotic minor liver resection was performed in 33 patients with a diagnosis of HCC (23). The procedures performed were 12 left lateral sectionectomies, 10 wedge resections, 7 segmentectomies and 4 bisegmentectomies. The mean operative time was 202.7 min and the mean blood loss was 373.4 mL. The complication and mortality rate were 7.1% and 0% respectively, with a negative resection margin of 93%. The authors concluded that robot-assistance is not only feasible and safe, but also does not increase tumor dissemination.
Tranchart et al. compared 26 cases of robotic minor hepatectomy performed in an Italian center, to 26 cases of laparoscopic minor hepatectomy performed in a French center (38). Interestingly, the authors report 42% portal triad clamping in the robotic group versus 0% in the laparoscopic group. This was attributed to the surgeons’ preference, which differed in the two centers. Although the two techniques had similar outcomes, the use of the robot seemed to facilitate the resection of posterior and superior liver tumors, especially when atypical resection was required.
There are several other reported cases describing robotic minor liver resections (6,25,26,44). Their results are in agreement with the aforementioned studies. Overall, postoperative outcomes are comparable to laparoscopy and the available short-term oncologic outcomes are encouraging. Robotic-assistance could definitely provide an advantage in the most complex cases, in posterior/superior segments and parenchyma-sparing resections. The results of the most important series of major hepatectomies are summarized in Tables 2 and 3.

Full table

Full table
Conclusions
In the last 20 years, minimally invasive surgery has gained a growing role in liver resections. It is now considered an option for the resection of the anterior and left lateral segments. This approach is also used in few highly specialized centers for major hepatectomies. Due to the limitations of the technique, laparoscopy is still not considered ideal for routine major hepatectomies, extended hepatectomies and for cases at high risk for bleeding.
Robotic surgery has the potential to overcome some of the limits of laparoscopy and, in past years, its range of applications in this field has quickly expanded. Major hepatectomies, extended right, extended left, posterior segments and living donor hepatectomies have been described in the literature (4,20,25,38,41,43,45,46). Perioperative and postoperative outcomes, as well as oncologic efficacy, are not inferior to open or laparoscopic surgery. This approach especially facilitates certain steps of the procedure, such as dissection of the hepatic hilum and hepatocaval plane, mobilization of the liver attachments, biliary anastomosis and suturing for bleeding management during the parenchymal transection. Furthermore, the robotic platform allows for easier integration of new technologies, such as the recently introduced near-infrared fluorescence, for vascular and biliary identification. Augmented reality, image-guided surgery and 3D ultrasound instruments with integrated probes for section margin assessment are all implementations, that in the near future will not only make the complex resections safer and more efficient, but also the routine resections.
There are no large, prospective studies regarding robotic hepatectomies published to date. Further investigation and multicenter trials are needed to validate the current promising results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-56. [Crossref] [PubMed]
- Milone L, Daskalaki D, Fernandes E, et al. State of the art in robotic hepatobiliary surgery. World J Surg 2013;37:2747-55. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery 2011;149:29-39. [Crossref] [PubMed]
- Soubrane O, Goumard C, Laurent A, et al. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB (Oxford) 2014;16:357-65. [Crossref] [PubMed]
- Packiam V, Bartlett DL, Tohme S, et al. Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg 2012;16:2233-8. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Bhojani FD, Fox A, Pitzul K, et al. Clinical and economic comparison of laparoscopic to open liver resections using a 2-to-1 matched pair analysis: an institutional experience. J Am Coll Surg 2012;214:184-95. [Crossref] [PubMed]
- Jackson NR, Hauch A, Hu T, et al. The Safety and Efficacy of Approaches to Liver Resection: A Meta-Analysis. JSLS 2015;19:e2014.00186.
- Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 2016;30:862-75. [Crossref] [PubMed]
- Vanounou T, Steel JL, Nguyen KT, et al. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol 2010;17:998-1009. [Crossref] [PubMed]
- Memeo R, de’Angelis N, Compagnon P, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg 2014;38:2919-26. [Crossref] [PubMed]
- Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 2010;211:16-23. [Crossref] [PubMed]
- Vibert E, Perniceni T, Levard H, et al. Laparoscopic liver resection. Br J Surg 2006;93:67-72. [Crossref] [PubMed]
- Gobardhan PD, Subar D, Gayet B. Laparoscopic liver surgery: An overview of the literature and experiences of a single centre. Best Pract Res Clin Gastroenterol 2014;28:111-21. [Crossref] [PubMed]
- Ocuin LM, Tsung A. Robotic liver resection for malignancy: Current status, oncologic outcomes, comparison to laparoscopy, and future applications. J Surg Oncol 2015;112:295-301. [Crossref] [PubMed]
- Ballantyne GH. The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 2002;12:1-5. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Giulianotti PC, Sbrana F, Coratti A, et al. Totally robotic right hepatectomy: surgical technique and outcomes. Arch Surg 2011;146:844-50. [Crossref] [PubMed]
- Choi GH, Choi SH, Kim SH, et al. Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc 2012;26:2247-58. [Crossref] [PubMed]
- Wu YM, Hu RH, Lai HS, et al. Robotic-assisted minimally invasive liver resection. Asian J Surg 2014;37:53-7. [Crossref] [PubMed]
- Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 2013;205:697-702. [Crossref] [PubMed]
- Troisi RI, Patriti A, Montalti R, et al. Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int J Med Robot 2013;9:160-6. [Crossref] [PubMed]
- Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 2011;253:342-8. [Crossref] [PubMed]
- Berber E, Akyildiz HY, Aucejo F, et al. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford) 2010;12:583-6. [Crossref] [PubMed]
- Koffron AJ, Auffenberg G, Kung R, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007;246:385-92; discussion 392-4. [Crossref] [PubMed]
- Abood GJ, Tsung A. Robot-assisted surgery: improved tool for major liver resections? J Hepatobiliary Pancreat Sci 2013;20:151-6. [Crossref] [PubMed]
- Flower RW, Hochheimer BF. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med J 1976;138:33-42. [PubMed]
- Hoekstra LT, de Graaf W, Nibourg GA, et al. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg 2013;257:27-36. [Crossref] [PubMed]
- Daskalaki D, Aguilera F, Patton K, et al. Fluorescence in robotic surgery. J Surg Oncol 2015;112:250-6. [Crossref] [PubMed]
- Daskalaki D, Fernandes E, Wang X, et al. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov 2014;21:615-21. [Crossref] [PubMed]
- Chung JJ, Kim MJ, Kim KW. Mangafodipir trisodium-enhanced MRI for the detection and characterization of focal hepatic lesions: is delayed imaging useful? J Magn Reson Imaging 2006;23:706-11. [Crossref] [PubMed]
- Perrenot C, Perez M, Tran N, et al. The virtual reality simulator dV-Trainer((R)) is a valid assessment tool for robotic surgical skills. Surg Endosc 2012;26:2587-93. [Crossref] [PubMed]
- Fisher RA, Dasgupta P, Mottrie A, et al. An over-view of robot assisted surgery curricula and the status of their validation. Int J Surg 2015;13:115-23. [Crossref] [PubMed]
- Fernandes E, Elli E, Giulianotti P. The role of the dual console in robotic surgical training. Surgery 2014;155:1-4. [Crossref] [PubMed]
- Li Z, Sun YM, Wu FX, et al. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol 2014;20:303-9. [Crossref] [PubMed]
- Tranchart H, Ceribelli C, Ferretti S, et al. Traditional versus robot-assisted full laparoscopic liver resection: a matched-pair comparative study. World J Surg 2014;38:2904-9. [Crossref] [PubMed]
- Casciola L, Patriti A, Ceccarelli G, et al. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 2011;25:3815-24. [Crossref] [PubMed]
- Chan OC, Tang CN, Lai EC, et al. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci 2011;18:471-80. [Crossref] [PubMed]
- Spampinato MG, Coratti A, Bianco L, et al. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc 2014;28:2973-9. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic extended right hepatectomy with biliary reconstruction. J Laparoendosc Adv Surg Tech A 2010;20:159-63. [Crossref] [PubMed]
- Chen KH, Chen SD, Chen YD, et al. Robotic left hepatectomy with revision of hepaticojejunostomy. Asian J Surg 2014;37:106-9. [Crossref] [PubMed]
- Patriti A, Cipriani F, Ratti F, et al. Robot-assisted versus open liver resection in the right posterior section. JSLS 2014;18. pii: e2014.00040.
- Tzvetanov I, Bejarano-Pineda L, Giulianotti PC, et al. State of the art of robotic surgery in organ transplantation. World J Surg 2013;37:2791-9. [Crossref] [PubMed]
- Giulianotti PC, Tzvetanov I, Jeon H, et al. Robot-assisted right lobe donor hepatectomy. Transpl Int 2012;25:e5-9. [Crossref] [PubMed]


