
International assessment and validation of the prognostic role of lymph node ratio in patients with resected pancreatic head ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common primary cancer of the pancreas and its incidence is steadily increasing with 10 to 15 new cases per 100,000 person-years in Western countries (1-4). Long-term outcome of PDAC remains poor (overall 3-year survival 6%) (5). Nevertheless, improved 3-year survival up to 63% has recently been reported by combining complete surgical resection and potent adjuvant chemotherapy (6).
A careful patient selection and decision-making are of utmost importance to achieve such results. Specific factors and biomarkers are mandatory to help clinicians precisely estimate the outcome of individual patients with PDAC. Several factors such as preoperative CA 19-9, lymph node involvement, or status of resection margin have been shown to be independent predictors of overall survival (OS) in PDAC patients (7-10).
Recently, lymph node ratio (LNR) defined as the number of positive lymph nodes divided by the number of collected lymph nodes during surgery was found to be a promising predictor of survival for different types of cancer (11-14). A few studies have also assessed its prognostic role in pancreas cancer (15-17). Some studies found that LNR was a better predictor of survival compared to lymph node involvement (pN stage) (15,16) and that it could also stratify survival in patients with similar lymph node involvement (16). Predominantly, single-center studies including different histological subtypes of pancreatic cancer were published, which impairs the generalizability of their findings (15,17). Moreover, LNR has not been assessed in the context of the new 8th edition of the tumor-node-metastasis (TNM) classification used since 2018 with the creation of the pN2 stage (18).
The present study aimed to assess the prognostic role of LNR predicting OS in patients undergoing curative surgical resection for PDAC in six high-volume centers and its relation with lymph node involvement. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-99/rc).
Methods
Consecutive patients with resectable PDAC who underwent upfront curative pancreatoduodenectomy (PD) from January 1st, 2000 to December 31st, 2017 were considered. Inclusion criteria were age >18 years and patients who did not refuse to have their personal data used for research. Patients with missing data regarding the number of collected lymph nodes or the number of positive lymph nodes were excluded, as well as patients with pre- or intraoperative metastases, with R2 resections, and who died during the first 90 postoperative days. Patients with other tumor types than PDAC such as duodenal cancers, distal cholangiocarcinomas, or ampullary tumors were not considered in order to minimize heterogeneity.
Diagnosis of PDAC was based on the pathology report of the PD specimen. Tumors were classified and staged according to the 8th edition of the TNM classification (AJCC staging system) (18). As all patients were operated before the publication of the TNM 8th edition, their tumor stages were regraded accordingly. Standard (not extended) lymphadenectomy during PD was performed in order to harvest at least 15 lymph nodes (19). Either pylorus-preserving or classic PD were realized according to local preferences. If needed, vascular resection (superior mesenteric vein, portal vein, superior mesenteric artery, hepatic artery) was performed. Resection margin status was based on the 1-mm rule as defined by the British Royal College of Pathologists (20).
Six tertiary international centers (five in Europe and one in the United States) participated to this study: Department of Visceral Surgery, Lausanne University Hospital CHUV (Lausanne, Switzerland), Division of Hepatobiliary and Pancreatic Surgery, Carolinas Medical Center (Charlotte, NC, USA), Pancreatic Surgery Section, Humanitas Cancer Center (Milan, Italy), Department of Digestive Surgery, Edouard Herriot Hospital (Lyon, France), Department of Surgery, Amsterdam University Medical Centers (Amsterdam, The Netherlands) and Department of Surgery, Leiden University Medical Center (Leiden, The Netherlands).
Lymph node involvement and LNR
Involvement of lymph nodes was defined based on pathological results. Of note, patients with direct lymph node invasion by the tumor were graded as pN1 as recommended by the British Royal College of Pathologists. Patients with lymph node involvement were classified as pN1 if 1–3 lymph nodes were positive and pN2 if ≥4 lymph nodes were positive (18). LNR was calculated with the following formula: number of positive lymph nodes divided by number of harvested lymph nodes (0≤ LNR ≤1).
The primary end point of the study was OS. OS was calculated from the date of PD until date of death (from any cause) or last follow-up. If patients were not dead at the time of survival analysis, the date of last-follow-up was censored.
Recurrence was defined as the occurrence of new tumoral disease, i.e., as regrowth of the primary tumor and/or lymph node recurrence after R0 resection, which was confirmed radiologically and/or based on pathological assessment. In R1 patients, recurrence was considered as progression of remnant microscopic disease. Recurrences were subdivided into local (resection site, pancreas remnant, local vascular vessels, local lymph nodes) or distant (metastasis).
Postoperative complications were defined according to Clavien classification as morbidity during the hospital stay or within the first 90 postoperative days (21). Pancreatic fistula, delayed gastric emptying, and postoperative hemorrhage were defined according to the International Study Group of Pancreatic Surgery (22-24).
During the first two years after PD, follow-up was scheduled every six months with CA 19-9 dosage and imaging [computed tomography (CT) or magnetic resonance imaging (MRI)]. Adjuvant treatment after PD for PDAC became the standard of care during the study period. At the beginning, in the early 2000s, adjuvant treatment was provided selectively to patients with lymph node involvement or positive resection margins.
Statistics
Categorical variables were presented as number with percentage and continuous variables as median with interquartile range (IQR). Median follow-up was calculated using the inverse Kaplan-Meier method. Survivals were calculated and represented using Kaplan-Meier graphs. Median OS was presented with 95% confidence interval (CI). Survival curves were compared using log-rank test, stratified if needed. To define independent factors of OS, multivariable Cox regressions (proportional hazards models) were performed to adjust for potential variables associated with prognosis. To avoid the risk of collinearity in the Cox analysis, the variable lymph node involvement (pN stage) was not introduced in the analysis as it is related to LNR. Similarly, tumor size was not considered as it defines pT stage. Only items with P value <1% on univariable analysis were included in the multivariable analysis. The significance threshold was defined as 5%. All tests were two-sided. LNR was analyzed as a categorical and a continuous variable. LNR threshold was defined using the maximal Youden index predictive of 2-year OS (cutoff to obtain the highest sensitivity and specificity) using a receiver operating characteristic (ROC) curve. The Youden index was calculated as sensitivity + specificity – 1. Area under the curve (AUC) was calculated with the C-statistic index. Accuracy of the prognostic value of LNR for OS over time was evaluated using a time-dependent AUC plot that was derived from several ROC curves. Inverse probabilities of censoring weights were calculated from a Kaplan-Meier estimator. SPSS Statistics for Mac OS X, version 25 (IBM Corp., Armonk, NY, USA) and R for Mac OS X, version 4.0.2 (R Foundation for Statistical Computing) were used for all statistical analyses.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee (#2017-1169) and individual consent for this retrospective analysis was waived.
Results
Patients
There were 1,513 patients from six tertiary centers who underwent upfront surgery for PDAC during the study period. Forty-one patients (2.7%) had missing data regarding the number of collected or positive lymph nodes, 38 metastases (2.5%), 26 R2 resections (1.7%), and 81 died perioperatively (Clavien grade V) during the first 90 postoperative days (5.8%, 30-day mortality: 47/1,513=3.1%), leaving a final cohort of 1,327 patients. These data are summarized in the flow chart of the study (Figure 1).
Median age of the entire cohort was 67 (IQR: 59–74) years, 48% (631/1,327) of patients were women, and 679 patients (51%) had at least one postoperative complication. Delayed gastric emptying occurred in 269 patients (20%), pancreatic fistula in 158 patients (12%), and hemorrhage in 107 patients (8%). Median OS and disease-free survival were 28 months (95% CI: 25–31 months) and 15 months (95% CI: 14–16 months) with a median follow-up of 58 months, respectively. Recurrences happened in 756 patients (57%) during the same median follow-up. Adjuvant exclusive chemotherapy was given in 710 patients (54%), adjuvant exclusive radiotherapy in 12 patients (1%), and combination of radiotherapy and chemotherapy in 176 patients (13%). Seventy-five percent of patients who had adjuvant chemotherapy alone or with radiotherapy (665/886) received gemcitabine, 11% gemcitabine combined with another drug (capecitabine, oxaliplatine, or FOLFIRINOX, 97/886), 2% (19/886) FOLFIRINOX, and 2% (19/886) capecitabine, FOLFOX, FOLFIRI, or 5-fluorouracil. Data regarding regimens of chemotherapy were missing for 86 patients (10%). Table 1 shows the demographics and the surgical and pathological details of the patients.
Table 1
| Characteristics | Median or number | IQR or percentage |
|---|---|---|
| Age, years | 67 | 59–74 |
| Sex (women) | 631 | 48 |
| Body-mass index, kg/m2 | 24.3 | 21.8–27.1 |
| Active smoker | 137 | 10 |
| Pre-existing diabetes | 167 | 13 |
| Jaundice | 939 | 71 |
| Preoperative biliary stenting | 809 | 61 |
| ASA score I–II | 831 | 63 |
| Highest CA 19-9, U/mL | 161 | 35–552 |
| Tumor size on pathology, mm | 30 | 22–38 |
| pT stage 1–2 | 267 | 20 |
| pN+ stage | 1,026 | 77 |
| pN1 | 561 | 42 |
| pN2 | 465 | 35 |
| Harvested lymph nodes | 18 | 12–24 |
| Positive lymph nodes | 3 | 2–6 |
| Lymph node ratio | 0.143 | 0.042–0.318 |
| Vascular invasion (V1)a | 544 | 41 |
| Lymphatic invasion (L1) | 561 | 43 |
| Perinervous invasion (Pn1) | 943 | 71 |
| Tumor grade | ||
| G1 | 118 | 9 |
| G2 | 650 | 49 |
| G3 | 534 | 40 |
| G4 | 25 | 2 |
| Resection margin status R0 | 756 | 57 |
| Pylorus-preserving Whipple | 1,001 | 75 |
| PJ anastomosis | 930 | 71 |
| Venous resection | 262 | 20 |
| Arterial resection | 9 | 1 |
| Operation time, min | 337 | 266–435 |
| Intraoperative blood loss, mL | 500 | 300–850 |
| Intraoperative blood transfusion | 201 | 15 |
Jaundice was defined as a clinical diagnosis. a, defined as microvascular invasion on pathology. IQR, interquartile range; ASA, American Society of Anesthesiologists; CA, carbohydrate antigen; PJ, pancreaticojejunal.
Lymph node involvement (pN+) was present in 1,026 patients (77%). Of those, 561 patients were staged pN1 (55%) and 465 pN2 (45%). Median number of harvested lymph nodes and positive lymph nodes were 18 (IQR: 12–24) and 3 (IQR: 2–6), respectively.
LNR
Median LNR was 0.143 (IQR: 0.042–0.318) in the entire cohort and 0.214 (IQR: 0.105–0.364) in pN+ patients. In pN1 patients (n=561) median LNR was 0.115 (IQR: 0.071–0.188) and in pN2 patients (n=465) median LNR was 0.333 (IQR: 0.250–0.500). The LNR threshold with the highest Youden index (best sensitivity and specificity) predictive of OS >24 months was 0.225 in the entire cohort (C-index: 0.622, sensitivity: 44% and specificity: 74%) and in pN+ patients (C-index: 0.570, sensitivity: 53% and specificity: 62%).
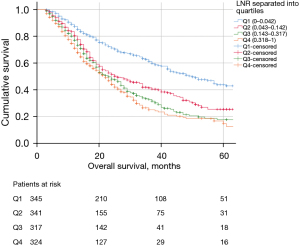
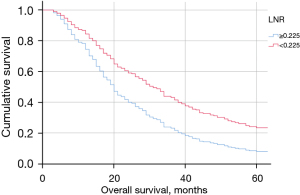
Patients with LNR ≥0.225 had worse OS compared to patients with LNR <0.225 (Figure 2A). Similar results were found in patients with lymph node involvement (pN+ patients) as shown in Figure 2B. If LNR is subdivided into quartiles (Q1: 0–0.042, Q2: 0.043–0.142, Q3: 0.143–0.317, Q4: 0.318–1), OS was significantly better in the first quartile (Figure 3). Median OS diminished from 50 months for patients in Q1 to Q22 months for patients in Q4.
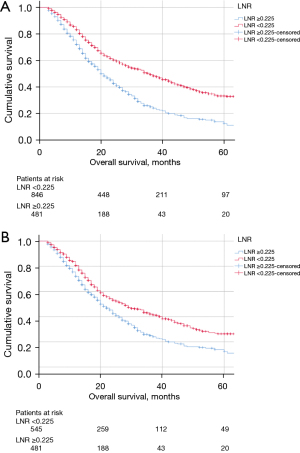
OS of patients with LNR ≥0.225 and LNR <0.225 stratified by American Society of Anesthesiologists (ASA) score remained significantly different (P<0.001). Similar results were found when stratified individually by tumor size, tumor differentiation, and adjuvant chemotherapy (all with P<0.001). After adjusting for all the above variables in a Cox model, LNR remained a significant predictor of OS (Figure 4).
In pN1 patients, OS curves were similar between LNR ≥0.225 and LNR <0.225 (Figure 5A). On the contrary in pN2 patients, LNR permitted to stratify the OS (Figure 5B). On multivariable analysis, LNR was an independent predictor of OS in the subgroup of N2 patients [hazard ratio (HR) =1.5; 95% CI: 1.0–2.1; P=0.045], along with preoperative stenting (HR =1.6; 95% CI: 1.2–2.2; P=0.003) and adjuvant chemotherapy (HR =2.0; 95% CI: 1.4–2.9; P<0.001). Of note, median OS was shorter for the group with >6 positive lymph nodes compared to the group with 4–6 positive lymph nodes (18 months, 95% CI: 16–20 vs. 24 months, 95% CI: 19–29, P=0.005). The cross-tabulation (Table 2) illustrates that LNR can substratify pN2 patients depending on the number of positive lymph nodes. Figure 6 shows the discriminative accuracy of LNR to predict OS over time compared to pN stage (i.e., pN0, pN1, and pN2). LNR displayed higher AUC over time compared to pN stage, which shows better discriminative power for LNR with regards to OS. In the subgroup of patients with harvested lymph nodes ≥15 and <15, patients with LNR ≥0.225 had worse median OS compared to patients with LNR <0.225 (Table 3).
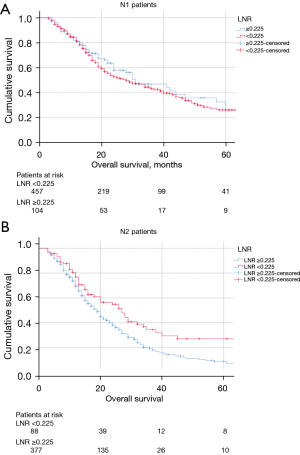
Table 2
| LNR <0.225 | LNR ≥0.225 | P value | |
|---|---|---|---|
| pN1 patients | 31 [19–43] | 28 [23–34] | 0.309 |
| positive LN: 1–2 | 29 [23–35] | 30 [23–37] | 0.931 |
| positive LN: 3 | 41 [25–57] | 27 [19–35] | 0.234 |
| pN2 patients | 27 [19–35] | 19 [17–21] | 0.003* |
| positive LN: 4–6 | 28 [25–31] | 20 [16–24] | 0.033* |
| positive LN >6 | 20 [14–26] | 18 [16–20] | 0.024* |
*, significant values. OS, overall survival; CI, confidence interval; LN, lymph nodes; LNR, lymph node ratio.
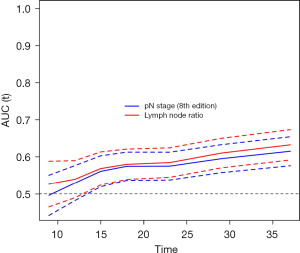
Table 3
| LNR <0.225 | LNR ≥0.225 | P value | |
|---|---|---|---|
| 0< Harvested LN <15 | 43 [35–51] | 24 [20–28] | <0.001* |
| Harvested LN ≥15 | 29 [23–35] | 18 [15–21] | <0.001* |
*, significant values. The cut-off of 15 corresponds to the recommended minimal number of collected lymph nodes according to the British Royal College of Pathology. OS, overall survival; CI, confidence interval; LN, lymph nodes; LNR, lymph node ratio.
LNR and recurrence
Patients with recurrence (n=756, 57%, median LNR: 0.182, IQR 0.071–0.333) had higher median LNR compared to patients without recurrence (n=571, 43%, median LNR 0.085, IQR: 0–0.250, P<0.001).
Regarding the pattern of recurrence, 155 patients (21%) had local recurrences, 352 (46%) distant metastases, and 236 (31%) both local and distant recurrences (13 missing data, 2%). Patients with local and distant recurrences had similar median LNR (0.143, IQR: 0.063–0.286 vs. 0.136, IQR: 0.038–0.300, P=0.358).
Predictive factors of survival
Table 4 summarizes the uni- and multivariable Cox analyses of predictive factors of OS. Independent factors of OS on multivariable analysis were ASA score, LNR, tumor differentiation, and adjuvant chemotherapy. Similar results were found for pN+ patients (Table 5).
Table 4
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | 1.0 (1.0–1.0) | 0.021 | 1.0 (1.0–1.0) | 0.950 | |
| Body-mass index, kg/m2 | 1.0 (1.0–1.0) | 0.973 | – | – | |
| Pre-existing diabetesa | 1.3 (1.0–1.6) | 0.020 | 0.8 (0.6–1.1) | 0.228 | |
| Jaundicea | 1.0 (0.8–1.2) | 0.745 | – | – | |
| Preoperative biliary stentinga | 1.0 (0.9–1.2) | 0.723 | – | – | |
| ASA score (ref: ASA 1, HR: 1)a | |||||
| ASA 2 | 2.1 (1.3–3.4) | 0.002 | 2.5 (1.2–4.9) | 0.011* | |
| ASA 3 | 1.7 (1.1–2.7) | 0.013 | 2.5 (1.4–4.3) | 0.002* | |
| ASA 4 | 1.6 (1.0–2.4) | 0.051 | 2.0 (1.1–3.5) | 0.020* | |
| Preoperative CA 19-9, U/mL | 1.0 (1.0–1.0) | <0.001 | 1.0 (1.0–1.0) | 0.098 | |
| pT stage (ref: pT1, HR: 1)a | |||||
| pT2 | 1.9 (1.2–3.1) | 0.012 | 1.8 (0.5–6.4) | 0.344 | |
| pT3 | 1.5 (1.0–2.3) | 0.048 | 1.1 (0.5–2.6) | 0.758 | |
| pT4 | 1.2 (0.8–1.7) | 0.057 | 1.0 (0.5–2.1) | 0.961 | |
| LNRb | 3.1 (2.3–4.2) | <0.001 | 5.5 (3.1–9.9) | <0.001* | |
| Vascular invasion (V1)a,c | 1.2 (1.1–1.4) | 0.007 | 1.3 (1.0–1.7) | 0.073 | |
| Lymphatic invasion (L) | 0.9 (0.8–1.1) | 0.484 | – | – | |
| Perineural invasion (Pn) | 0.8 (0.7–1.0) | 0.042 | 0.8 (0.7–1.1) | 0.121 | |
| Differentiation (ref: G1, HR: 1)a | |||||
| G2 | 2.2 (1.2–4.0) | 0.007 | 3.1 (1.5–6.6) | 0.003* | |
| G3 | 2.3 (1.3–4.0) | 0.003 | 2.9 (1.5–5.6) | 0.001* | |
| G4 | 1.4 (0.8–2.4) | 0.052 | 2.0 (1.1–3.7) | 0.030* | |
| Margin status (ref: R0, HR: 1)a | |||||
| R1 | 1.7 (1.0–2.9) | 0.064 | 0.8 (0.3–2.1) | 0.703 | |
| Portal vein resectiona | 1.1 (1.0–1.4) | 0.148 | – | – | |
| Operation time | 1.0 (1.0–1.0) | <0.001 | 1.0 (1.0–1.0) | 0.813 | |
| Adjuvant chemotherapya | 1.2 (1.0–1.4) | 0.015 | 2.0 (1.5–2.6) | <0.001* | |
a, categorical variables; b, if analyzed as a categorical variable (≥0.225 or <0.225), multivariable HR for LNR was 1.7 (95% CI: 1.4–2.2, P<0.001); c, defined as microvascular invasion on pathology. *, significant values. All univariate P values <0.1 were included in the multivariate analysis. HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; ref, reference; CA, carbohydrate antigen; LNR, lymph node ratio.
Table 5
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | 1.0 (1.0–1.0) | 0.005* | 1.0 (1.0–1.0) | 0.966 | |
| Body-mass index, kg/m2 | 1.0 (1.0–1.0) | 0.757 | |||
| Pre-existing diabetesa | 0.8 (0.7–1.1) | 0.117 | |||
| Jaundicea | 0.9 (0.8–1.1) | 0.455 | |||
| Preoperative biliary stentinga | 0.9 (0.8–1.1) | 0.480 | |||
| ASA score (ref: ASA 1, HR: 1)a | |||||
| ASA 2 | 2.2 (1.3–3.8) | 0.003* | 2.3 (1.2–4.6) | 0.015* | |
| ASA 3 | 1.8 (1.1–2.8) | 0.019* | 2.1 (1.2–3.8) | 0.010* | |
| ASA 4 | 1.5 (0.9–2.4) | 0.077 | 1.7 (0.9–3.0) | 0.092 | |
| Preoperative CA 19-9, U/mL | 1.0 (1.0–1.0) | 0.015* | 1.0 (1.0–1.0) | 0.205 | |
| pT stage (ref: pT1, HR: 1)a | |||||
| pT2 | 1.7 (0.9–3.2) | 0.076 | 1.4 (0.5–3.9) | 0.535 | |
| pT3 | 1.5 (1.0–2.4) | 0.080 | 1.2 (0.6–2.4) | 0.670 | |
| pT4 | 1.2 (0.8–1.8) | 0.408 | |||
| LNRb | 1.9 (1.3–2.8) | <0.001* | 3.8 (2.2–6.6) | <0.001* | |
| Vascular invasion (V1)a,c | 1.0 (0.8–1.1) | 0.693 | |||
| Lymphatic invasion (L) | 1.0 (0.8–1.2) | 0.966 | |||
| Perineural invasion (Pn) | 0.9 (0.7–1.1) | 0.200 | |||
| Differentiation (ref: G1, HR: 1)a | |||||
| G2 | 2.3 (1.2–4.2) | 0.010* | 2.9 (1.4–5.8) | 0.003* | |
| G3 | 2.4 (1.4–4.4) | 0.003* | 2.8 (1.5–5.2) | 0.002* | |
| G4 | 1.6 (0.9–2.9) | 0.060 | 1.9 (1.0–3.5) | 0.040* | |
| Margin status (ref: R0, HR: 1)a | |||||
| R1 | 1.6 (0.9–2.8) | 0.082 | 1.3 (0.5–3.2) | 0.605 | |
| Portal vein resectiona | 1.0 (0.8–1.2) | 0.934 | |||
| Operation duration | 1.0 (1.0–1.0) | 0.001* | 1.0 (1.0–1.0) | 0.706 | |
| Adjuvant chemotherapya | 1.5 (1.2–1.8) | <0.001* | 2.2 (1.8–2.8) | <0.001* | |
*, significant values; a, categorical variables; b, if analyzed as a categorical variable (≥0.225 or <0.225), multivariable HR for LNR was 1.6 (95% CI: 1.3–2.0, P<0.001); c, defined as microvascular invasion on pathology (V1 according to TNM staging). All univariate P values <0.1 were included in the multivariate analysis. HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; ref, reference; CA, carbohydrate antigen; LNR, lymph node ratio.
Discussion
This study found that LNR was a strong independent predictor of OS (HR =5.5; 95% CI: 3.1–9.9) in patients with PDAC who underwent upfront surgery. Furthermore, LNR was predictive of OS in pN+ patients (especially in pN2 patients, ≥4 positive lymph nodes) and permitted to stratify the survival outcomes in this subgroup of patients.
On multivariable analysis, LNR was found to be the strongest predictive factor of OS in the entire cohort and in pN+ patients (Tables 4,5). Several studies also found LNR as a predictor of OS in pancreatic cancer (15-17,25-30). Most of the studies were either monocentric, with a small cohort size, or mixing different histological types of tumors (distal cholangiocarcinomas, ductal adenocarcinomas, or ampullary tumors) on the contrary of the present study that entails six international centers with a large cohort of patients with exclusively PDAC of the pancreatic head. Farid et al. found in their study evaluating 551 patients with periampullary cancers that LNR was significantly predictive of OS in patients with 4–5 positive lymph nodes and that there was a trend in patients with >6 positive lymph nodes (16). On the contrary, patients with 0–3 positive lymph nodes did not display different survival in the various LNR subgroups (0.01–0.2, 0.2–0.4, >0.4) (16). These results corroborate our findings. In the pN1 group, patients with LNR <0.225 and ≥0.225 had similar OS, whereas in pN2 patients (and in subgroups of pN2 patients, Table 2) a difference of OS was found in benefit of patients with LNR <0.225. Even though pN2 patients with a higher number of positive lymph nodes (>6 lymph nodes) had worse OS and could discriminate better than present TNM classification, subdivision of these groups with LNR permitted to further stratify OS. Pawlik et al. in a retrospective single-center analysis of 905 patients showed that LNR was able to stratify survival among all subsets of pN+ patients (positive lymph nodes: 1–4, 4–6, >6) (26). These findings only differ from the present study’s results with regards to patients with <4 positive lymph nodes.
Apart from LNR, ASA score, tumor grade, and adjuvant chemotherapy were found as independent predictors of OS in this cohort. All these factors have been shown in several studies to influence the outcomes of pancreatic cancer patients (17,26,30,31). Interestingly, pT stage based on tumor size and status of resection margin were not independent predictors of OS in this cohort. This highlights the fact that tumor size did not play a prognostic role in terms of survival in the present cohort. Similar results were found in an international validation study of the 8th edition of the AJCC staging system, where OS was not different across pT stages (defined according to the 7th and 8th TNM edition) in pN0 patients (10). The fact that the status of resection margin was not significant in this cohort can potentially be explained by the histology assessments that were made by various international pathologists in the different centers, by the rather low R0 rate (57%) and/or by the preponderant effect of lymph node involvement (present in 77% of the patients). Evaluation of surgical specimens by different pathologists can induce slight variations in the count of harvested or positive lymph nodes, which can affect the results. Additionally, even though all centers used the 1-mm definition for the resection margin status, some cases before 2009 (date of publication of the 1-mm definition by the British Royal College of Pathologists) were retrospectively graded, which can be challenging. Similarly to the results of this study, several articles recently showed that resection margin status was not a predictive factor of OS when lymph node involvement was present (32,33).
The present study found a threshold for LNR at 0.225. Of note, this threshold was associated with a rather low area under the curve (0.622). This threshold to predict OS >24 months was determined at one time point (24 months) and the related area under the curve reflects the discriminative power of this threshold that can be considered as relatively poor. Several cutoffs have been recently published. Tol et al. found in their retrospective study that the best LNR threshold for 3-year OS in PDAC patients was 0.18 (17). This threshold was found using the maximum likelihood estimate of the log rank. He et al. showed that the best LNR threshold was 0.17 defined using the maximal Youden index such as in this study (27). In two different studies, a predefined LNR threshold of 0.2 was used based on sensitivity analyses and literature data (15,16). Pawlik et al. stratified LNR into four predefined categories (26), whereas Song et al. used recursive partitioning analysis to find four classes (29). Lowder et al. separated LNR by deciles (28). To summarize, various methods and thresholds were used and found, but all studies showed a prognostic value of LNR. It is important to remember that dichotomization or categorization of time-to-event variables such as survival induces biases and that it is mainly done to have a clinical landmark. It is also worth noting that thresholds remain indicators and that they should not be seen as unchangeable or inflexible. Survival data should rather be interpreted as a continuum. For example, variables around the defined threshold might have very similar prognosis.
Subgroup analysis of pN1 and pN2 showed that LNR was able to stratify OS in pN2 patients. In pN2 patients, OS was indeed higher in patients with LNR <0.225, whereas both groups did not show an OS difference in pN1 patients. This result suggests that LNR is more specific in terms of survival prediction than pN stage according to the 8th TNM edition as also showed in Figure 6. LNR use permits to refine the prognosis prediction in patients with lymph node involvement. It should therefore be used in complement to pN staging to obtain a more precise survival prognosis. Another point not evaluated in this study is whether LNR remains a strong prognostic factor in patients who undergo neoadjuvant treatment. Future studies will need to assess these issues.
High LNR might have postoperative clinical implications. As high LNR suggests a higher risk of shorter survival, a more aggressive adjuvant chemotherapeutic regimen might be chosen, the chemotherapy duration could be lengthened, adjuvant radiotherapy could be used, or the follow-up with imaging could be performed at shorter intervals. All these potential strategies need to be assessed in prospective studies using a treatment or follow-up algorithm based on LNR.
Several limitations need to be addressed. Based on the retrospective study design, errors of data collection or data transcription might have occurred. Moreover, the study period is quite long, which can bring heterogeneity. The absence of standardization of surgical procedure, perioperative management, and pathological assessment between the various included centers also is a study limitation. Chemotherapy types and regimens were heterogeneous among centers. The defined LNR threshold might mathematically contains biases as dichotomization of time-to-event variables is usually not recommended. However, a threshold is important for clinical practice and for statistical reasons. The strength of this study lies in the pathological homogeneity of the cohort (only patients with PDAC without neoadjuvant treatment), in the large sample size, and in the international multicentricity.
In conclusion, this study was an international validation of the prognostic role of LNR in a large cohort of patients from five countries in Europe and the United States, allowing the results to be generalizable. LNR was found to be a strong independent predictor of OS in the present cohort of PDAC patients who underwent upfront surgery. LNR can even better stratify the prognosis of pN2 patients and should be routinely described in pathology reports and used in clinical practice.
Acknowledgments
This article was presented in parts at the Annual Meeting of the Swiss Congress of Surgery (September 2020) and at the World Congress of the IHPBA (November 2020).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-99/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-99/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-99/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318-48. [Crossref] [PubMed]
- Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer 2020;125:83-93. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:934-47. [Crossref] [PubMed]
- Fristrup C, Detlefsen S, Hansen CP, et al. Danish Pancreatic Cancer Database. Clin Epidemiol 2016;8:645-8. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Delpero JR, Jeune F, Bachellier P, et al. Prognostic Value of Resection Margin Involvement After Pancreaticoduodenectomy for Ductal Adenocarcinoma: Updates From a French Prospective Multicenter Study. Ann Surg 2017;266:787-96. [Crossref] [PubMed]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74-85. [Crossref] [PubMed]
- Honselmann KC, Pergolini I, Castillo CF, et al. Timing But Not Patterns of Recurrence Is Different Between Node-negative and Node-positive Resected Pancreatic Cancer. Ann Surg 2020;272:357-65. [Crossref] [PubMed]
- van Roessel S, Kasumova GG, Verheij J, et al. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg 2018;153:e183617.
- Mansour J, Sagiv D, Alon E, et al. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol 2018;132:8-13. [Crossref] [PubMed]
- Yamashita K, Hosoda K, Ema A, et al. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur J Surg Oncol 2016;42:1253-60. [Crossref] [PubMed]
- Zhao Y, Li G, Zheng D, et al. The prognostic value of lymph node ratio and log odds of positive lymph nodes in patients with lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;153:702-9.e1. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Pomianowska E, Westgaard A, Mathisen Ø, et al. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol 2013;20:233-41. [Crossref] [PubMed]
- Farid SG, Falk GA, Joyce D, et al. Prognostic value of the lymph node ratio after resection of periampullary carcinomas. HPB (Oxford) 2014;16:582-91. [Crossref] [PubMed]
- Tol JA, Brosens LA, van Dieren S, et al. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br J Surg 2015;102:237-45. [Crossref] [PubMed]
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591-600. [Crossref] [PubMed]
- Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009;55:277-83. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Falconi M, Crippa S, Domínguez I, et al. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol 2008;15:3178-86. [Crossref] [PubMed]
- Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610-8. [Crossref] [PubMed]
- He C, Mao Y, Wang J, et al. Nomograms predict long-term survival for patients with periampullary adenocarcinoma after pancreatoduodenectomy. BMC Cancer 2018;18:327. [Crossref] [PubMed]
- Lowder CY, Metkus J, Epstein J, et al. Clinical Implications of Extensive Lymph Node Metastases for Resected Pancreatic Cancer. Ann Surg Oncol 2018;25:4004-11. [Crossref] [PubMed]
- Song Y, Chen Z, Chen L, et al. A Refined Staging Model for Resectable Pancreatic Ductal Adenocarcinoma Incorporating Examined Lymph Nodes, Location of Tumor and Positive Lymph Nodes Ratio. J Cancer 2018;9:3507-14. [Crossref] [PubMed]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [Crossref] [PubMed]
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311-9. [Crossref] [PubMed]
- Tummers WS, Groen JV, Sibinga Mulder BG, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg 2019;106:1055-65. [Crossref] [PubMed]
- Joliat GR, Allemann P, Labgaa I, et al. Prognostic value of positive histological margins in patients with pancreatic head ductal adenocarcinoma and lymph node involvement: an international multicentric study. HPB (Oxford) 2021;23:379-86. [Crossref] [PubMed]



