The veno-venous bypass in liver transplantation: an unfinished product
Introduction
Our team favors preservation of the native inferior vena cava (IVC) and (early whenever feasible) temporary porto-caval shunt during liver transplantation (LT). Nevertheless, we still resort to resection of the IVC in the following situations: vicinity of a liver tumor or technical constraints such as huge segment 1 or huge liver (such as in polycystic liver disease). In these type of situations, the percutaneous veno-venous bypass (VVB) (1) including temporary porto-caval shunt is installed whenever one of the following criteria is fulfilled: recipient age ≥65 years, pre-existing kidney dysfunction, coronary disease history, severe intraoperative bleeding due to portal hypertension, and intolerance to IVC clamping despite adequate cardiac preload management. In addition, VVB might be used as a “safety net” during training of youngest LT surgeons without excluding further IVC preservation. With these indications, VVB was used in 34/384 (8.9%) LTs performed at our center during the last 4 years. We describe here the decompression of the porto-mesenteric compartment via a patent para-umbilical vein in a patient needing a VVB during orthotopic LT.
Case presentation
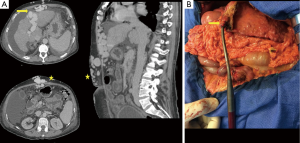
A 54-year-old male underwent evaluation for LT for end-stage alcoholic liver disease (Child C score and MELD score =32). He had severe portal hypertension including thrombopenia (platelet count =28×103/mL), massive intractable ascites, splenomegaly, and a past history of repeated rupture of esophageal varices. The patient had no cardiac comorbidity or renal dysfunction estimated (glomerular filtration =72 mL/min/1.73 m2, serum creatinine =74 µmol/L). Pre-transplant workup showed a large patent para-umbilical vein (30 mm in diameter on computed tomography) with hepatofugal right portal vein flow and massive collateral veins in the abdominal wall (Figure 1A).
Surgical technique
At transplant, a femoro-axillary percutaneous VVB was installed [15 F cannulas, centrifugal constrained vortex pump head (Medtronic Biomedicus, Inc, MN, USA)] prior to abdominal opening to decompress massive collateral veins in the abdominal wall. Surgery was then started through a bilateral subcostal abdominal incision. The round ligament was stapled while saving a long stump on the liver side. The latter was catheterized with a 15-F cannula and connected to the VVB (Figure 1B).
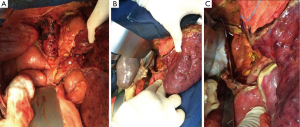
Adjustments in VVB bypass flow were made frequently upon anesthetist’s request and according to patient hemodynamic parameters. The bypass flow was set between 2 and 3 L/min. Cardiopulmonary parameters including bypass pump flow, cardiac output and mean arterial pressure were monitored during the surgery. As a consequence, the use of this technique was associated with stable cardiac output (14.9 to 17.6 L/min), absence of splanchnic congestion and edema, and without significant changes in haemodynamics [noradrenaline (<0.1 mg/hr), no need for adrenaline] and biological parameters [lactatemia (1.2 to 2.5 mmol/L)], and body temperature (35.9 to 36.3 °C). Apart from the portal vein, the native liver was then totally disconnected. The native IVC was left in place after stapling of the main hepatic veins and ligation of all smaller effluent veins (Figure 2A). The severe atrophy of the native liver (1,127 g) allowed to place the whole liver graft (1,240 g) in the “liver fossa” while maintaining the native liver pulled toward the left side and connected to the biopump. This maneuver maintained splanchnic venous decompression during latero-lateral cavo-caval anastomosis (Figure 2B,C). The cannula of the umbilical vein was then clamped and removed. The native portal vein was clamped and divided. Standard portal, arterial, and biliary reconstructions were then performed. No post-reperfusion syndrome occurred. The hemodynamic parameters remained stable with mean arterial and pulmonary pressures of 84 and 26 mmHg respectively. The cardiac index remained unchanged. The VVB was stopped and removed when the anesthesiologists confirmed hemodynamic stability.
Peri-operative course
Total bypass time was 282 minutes. The anhepatic time was 30 minutes, and the cold ischemia time was 7.5 hours. The operative time was 12 hours. The patient received 13 units of packed red blood cells, 13 units of fresh frozen plasma and 1 unit of platelet concentrate. The postoperative course was uneventful. Computed tomography scan at the seven postoperative days showed good inflow and outflow. There were no complications related to venous access techniques and the patient was discharged home on day 15 with normal liver and kidney function.
Discussion
When indicated, we consider the percutaneous VVB combined to porto-mesenteric decompression as optimal (1). In this case, the patient showed recanalization of the umbilical vein as his main spontaneous porto-systemic shunt. To obviate the development of splanchnic edema during the anhepatic phase of LT, we hypothesized that the presence of a widely patent para-umbilical vein could be used much easier and faster as vascular access to provide pump inflow than the portal vein. The above-described technique provides the following advantages in the peculiar setting of a patent para-umbilical vein (I) the use of the latter to decompress the portal system is straightforward, quasi immediate as it is done upon opening the abdominal wall, and optimizes the VVB flow early during the LT procedure; and (II) this technique avoids portal vein or inferior mesenteric vein dissection (and sacrificed upon VVB removal), and obviates the risk of bleeding from large vein collateral circulation encountered during their control. A patent para-umbilical vein has been reported in up to one third of patients with portal hypertension (2), and could be used whenever VVB is indicated during LT in this setting.
In the present case, the orthotopic positioning of the graft could be achieved whereas the portal decompression was left in place during the cavo-caval anastomosis. The atrophic native liver did not clutter up the operating field. Should the native liver not be atrophied, this could be removed before implantation of the graft. At this stage, the cannulation of the native portal vein could replace the para-umbilical vein cannulation if needed.
In summary, VVB using a patent para-umbilical vein during LT is a safe and simple technique and may be useful for patients with a large patent para-umbilical vein associated with reversed (hepatofugal) flow in the right portal vein upon Doppler ultrasound. This technical refinement obviates the dissection of the inferior mesenteric or portal vein, particularly in case of portal hypertension. More than 30 years after its first use in LT, VVB remains an “unfinished product” rather than anachronistic (3).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this article and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
References
- Oken AC, Frank SM, Merritt WT, et al. A new percutaneous technique for establishing venous bypass access in orthotopic liver transplantation. J Cardiothorac Vasc Anesth 1994;8:58-60. [Crossref] [PubMed]
- Sacerdoti D, Bolognesi M, Bombonato G, et al. Paraumbilical vein patency in cirrhosis: effects on hepatic hemodynamics evaluated by Doppler sonography. Hepatology 1995;22:1689-94. [PubMed]
- Denmark SW, Shaw BW Jr, Starzl TE, et al. Veno-venous bypass without systemic anticoagulation in canine and human liver transplantation. Surg Forum 1983;34:380-2. [PubMed]


